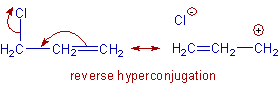The delocalization of σ-electrons or lone pair of electrons into adjacent π-orbital or p-orbital is called hyperconjugation.
It occurs due to overlapping of σ-bonding orbital or the orbital containing a lone pair with adjacent π-orbital or p-orbital.
It is also known as "no bond resonance" or "Baker-Nathan effect".
Conditions for hyperconjugation
* There must be an α-CH group or a lone pair on atom adjacent to sp2 hybrid carbon or other atoms like nitrogen, oxygen etc.
E.g., Alkenes, alkyl carbocations, alkyl free radicals, nitro compounds with α- hydrogen
ILLUSTRATION OF HYPERCONJUGATION
The displacement of σ-electrons towards the multiple bond occurs when there are hydrogens on the α-carbon (which is adjacent to the multiple bond). This results in the polarization of the multiple bond.
E.g. In propene, the σ-electrons of C-H bond of methyl group can be delocalized into the π-orbital of doubly bonded carbon as represented below.
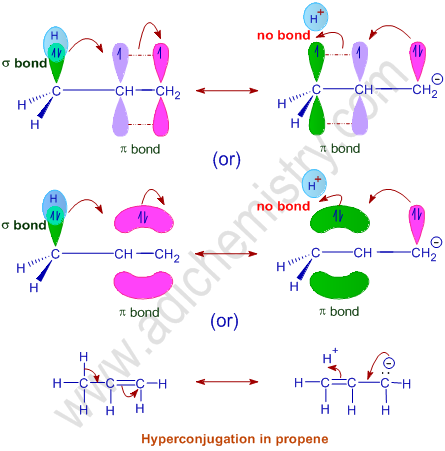
In the same way, the other hydrogens on the methyl group also participate in the hyperconjugation. This is possible due to free rotation of C-C bond so that the other C-H bonds can also participate in the hyperconjugation. Thus the propene molecule can show following resonance structures, which confer stability to it.
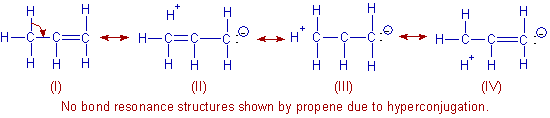
In the contributing structures: (II), (III) & (IV) of propene, there is NO bond between an α-carbon and one of the hydrogen atom. Hence the hyperconjugation is also known as "no bond resonance".
These equivalent contributing structures i.e., (II), (III) & (IV) are also polar in nature and hence are responsible for the dipole moment of propene (0.36 D).
The C-C bond lengths in propene are equal to 1.48. Its value is in between 1.54 (of C-C) and 1.34 (of C=C). It is because the bond order of C-C bonds is approximately 1.5 due to hyperconjugation.
This type of hyperconjugation is also referred to as sacrificial hyperconjugation since one bond is missing.
CONSEQUENCES & APPLICATIONS OF HYPERCONJUGATION
1) Stability of alkenes:
A general rule is that, the stability of alkenes increases with increase in the number of alkyl groups (containing hydrogens) on the double bond. It is due to increase in the number of contributing no bond resonance structures.
For example, 2-butene is more stable than 1-butene. This is because in 2-butene, there are six hydrogens involved in hyperconjugation whereas there are only two hydrogens involved in case of 1-butene. Hence the contributing structures in 2-butene are more and is more stable than 1-butene.
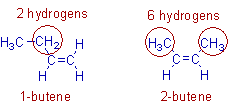
The increasing order of stability of alkenes with increases in the number of methyl groups on the double bond is depicted below.
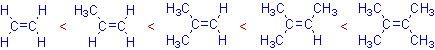
This order is supported by the heat of hydrogenation data of these alkenes. The values of heats of hydrogenation decrease with increase in the stability of alkenes.
Also the heats of formation of more substituted alkenes are higher than expected.
However it is important to note that the alkyl groups attached to the double bond must contain at least one hydrogen atom for hyperconjugation. For example, in case of the following alkene containing a tert-butyl group on doubly bonded carbon, the hyperconjugation is not possible.
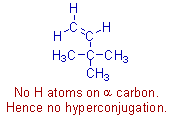
It is also important to note that the effect of hyperconjugation is stronger than the inductive effect.
For example, the positive inductive effect of ethyl group is stronger than that of methyl group. Hence based on inductive effect, 1-butene is expected to be more stable than propene.
However propene is more stable than 1-butene. This is because there are three hydrogens on α-methyl group involved in hyperconjugation. Whereas, in 1-butene there are only two hydrogen atoms on -CH2 group that can take part in hyperconjugation.
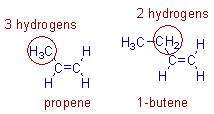
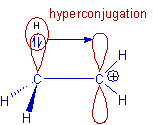
2) Stability of carbocations (carbonium ions):
The ethyl carbocation, CH3-CH2+ is more stable than the methyl carbocation, CH3+. This is because, the σ-electrons of the α-C-H bond in ethyl group are delocalized into the empty p-orbital of the positive carbon center and thus by giving rise to 'no bond resonance structures' as shown below. Whereas hyperconjugation is not possible in methyl carbocation and hence is less stable.
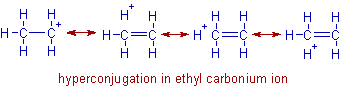
In general, the stability of carbonium ions increases with increase in the number of alkyl groups (containing hydrogen) attached to the positively charged carbon due to increase in the number of contributing structures to hyperconjugation.
Note: This type of hyperconjugation can also referred to as isovalent hyperconjugation since there is no decrease in the number bonds in the no bond resonance forms.
Thus the increasing order of stability of carbocations can be given as: methyl < primary < secondary < tertiary as depicted below:
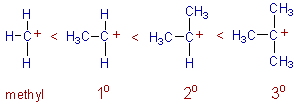
3) Stability of free radicals:
The stability of free radicals is influenced by hyperconjugation as in case of carbonium ions. The the σ-electrons of the α-C-H bond can be delocalized into the p-orbital of carbon containing an odd electron.
Due to hyperconjugation, the stability of free radicals also follow the same order as that of carbonium ions i.e., methyl < primary < secondary < tertiary.
4) Dipole moment & bond length:
* The dipole moment of the molecules is greatly affected due to hyperconjugation since the contributing structures show considerable polarity.
* The bond lengths are also altered due to change in the bond order during hyperconjugation. The single bond may get partial double bond character and vice versa.
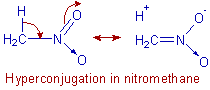
E.g. The observed dipole moment of nitro methane is greater than the calculated value due to hyperconjugation. The observed C –N bond length is also less than the expected value due to same reason.
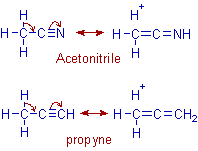
The same arguments can be applied to shortening of C-C bond adjacent to -C≡N in acetonitrile and also the C-C bond adjacent to the -C≡C in propyne. Also note that the observed dipole moments are again different from their expected values.
5) Reactivity & orientation of electrophilic substitution on benzene ring :
In Toluene, the methyl group releases electrons towards the benzene ring partly due to inductive effect and mainly due to hyperconjugation. Thus the reactivity of the ring towards electrophilic substitution increases and the substitution is directed at ortho and para postions to the methyl group.
The no bond resonance forms of toluene due to hyperconjugation are shown below.
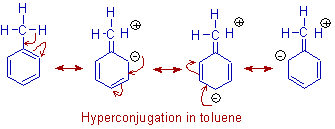
From the above diagram, it can be seen clearly that the electron density on benzene ring is increased especially at ortho and para positions.
Since the hyperconjugation overpowers the inductive effect, the substitution (e.g. nitration) on the following disubstituted benzene occurs ortho to the methyl group. In the tert-butyl group, there are no hydrogens on the carbon directly attached to the benzene ring. Hence it cannot involve in hyperconjugation.
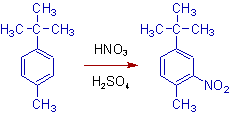
Also note that the tert-butyl group is bulky and hinders the approach of electrophile.
6) Anomeric effect :
The general tendency of anomeric substituents to prefer an axial position is called Anomeric effect.
For example, the α-methyl glucoside is more stable than the β-methyl glucoside due to hyperconjugation.
In α-methyl glucoside, the non bonding HOMO with a pair of electrons on the ring oxygen is antiperiplanar to the antibonding LUMO of C-O bond in methoxy group. This allows hyperconjugation between them and thus by stabilizing the α-form.
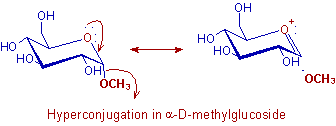
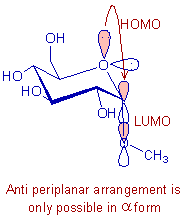
Whereas, in β-methyl glucoside the methoxy group is at equatorial position and cannot involve in hyperconjugation since it is not antiperiplanar to the lone pair on ring oxygen. Therefore β-methyl glucoside is less stable than the α-methyl glucoside.
7) Reverse hyperconjugation:
In case of α-halo alkenes, the delocalization of electrons occurs towards halogen group through hyperconjugative mechanism. It is referred to as reverse hyperconjugation. The dipole moments of α-halo alkenes are augmented due to this phenomenon.