(IIT JEE 1983)
1) electrovalent and covalent
2) electrovalent and coordinate covalent
3) electrovalent, covalent and coordinate covalent
4) covalent and coordinate covalent
Logic & Solution:
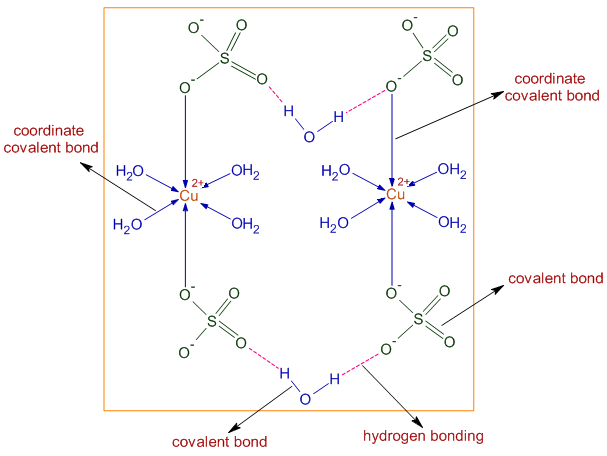
* Structure of CuSO4.5H2O in the solid state is shown below. The Cu2+ ions are attracted towards SO42- ions not only by ionic interactions (electrovalent) but also by coordinate covalent bonds. The Cu2+ ions form coordinate covalent bonds with water as well as sulfate ions.
There are covalent bonds in water and sulfate ions.

Conclusion:
The correct option is 3.
Note:
There is also hydrogen bonding between water and sulfate ions. Only four water molecules are forming dative bonds with Cu2+. The fifth molecules is involved in H-bonding.
(IIT JEE 1997)
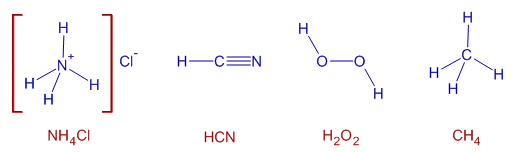
1) NH4Cl
2) HCN
3) H2O2
4) CH4
Logic & solution:
1) In NH4Cl, the N-H bond is polar due to appreciable electronegativity difference between N (3.0) and H (2.1).
2) In HCN, the H-C and C-N bonds are polar due to electronegativity difference between C (2.5) and N (3.0) for H-C bond as well as between C (2.5) and H (2.1) for C-N bond.
3) In H2O2, the O-H bond is polar due to electronegativity difference between O (3.5) and H (2.1). Whereas, O-O bond is non-polar.
4) In CH4, the C-H bonds are also polar.
Note: Even though, the C-H bonds have a little polarity, you might have seen that they are considered to be non-polar in the study of organic chemistry. It is a relative thing. They are less polar when compared to bonds formed by carbon with other atoms like: C-O, C-N, C-X etc. Hence C-H bonds are treated as non-polar.
But according to the tone of this question, C-H bonds are taken as polar since there is electronegativity difference.
Conclusion:
Option '3' is correct.
(IIT JEE 1996)
1) one σ and one π bonds
2) one σ and two π bonds
3) one σ and one and half π bonds
4) one σ bond
Logic & solution:
CaC2 can be divided into Ca2+ and C22- ions. The structure of C22- ion is shown below:
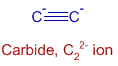
There is a triple bond between two carbons. Among three bonds, only one is sigma bond and the remaining two are pi bonds.
Conclusion:
Option '2' is correct.
(EAMCET 2002-E)
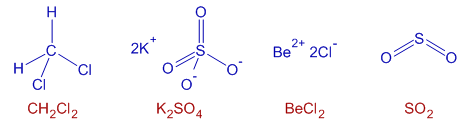
1) CH2Cl2
2) K2SO4
3) BeCl2
4) SO2
Logic & solution:
Only in K2SO4, there are ionic as well as covalent bonds. The K+ and SO42- ions are attracted by electrostatic forces. Sulfur atom has covalent bonds in sulfate ion.
Conclusion:
Option '2' is correct.
(IIT JEE 1980)
1) X+Y-
2) Y+X-
3) X-Y
4) X→Y
Logic & solution:
Strongly electropositive elements tend to lose electrons due to relatively low ionization potentials to form cations (like X+) and strongly electronegative elements tend to gain electrons to form anions (like Y-). Hence the bond formed between them is predominantly ionic in nature.
Conclusion:
Option '1' is correct.
(IIT JEE 1986)
1) Unequally shared between the two.
2) Transferred fully from one atom to another
3) Has identical spins.
4) Equally shared between the atoms.
Logic & solution:
Identical atoms possess same electronegativity values and hence share the electron pair equally by forming a non polar covalent bond.
Conclusion:
Option '4' is correct.
(IIT JEE 1986)
1) only ionic
2) covalent and coordinate
3) only covalent
4) covalent and ionic
Logic & solution:
Since nitrogen and oxygen are non metals, the bonds between them are NOT ionic.
The structure of N2O5 is given below. There are both covalent and coordinate covalent bonds.
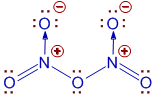
Conclusion:
Option '2' is correct.
1) ?
Author: Aditya vardhan Vutturi