| < Previous question | Next question > |
(IIT JEE 1979)
1) 22
2) 44
3) 66
4) 88
Logic:
Number of electrons in a molecule is equal to the sum of electrons in individual atoms in it.
# electrons in an atom is equal to its atomic number.
Solution:
In CO2, there are one carbon and two oxygen atoms and the number of electrons in them are:
In C ------> 6 electrons
In two O's -------> 2 x 8 electrons
Total in CO2 -------> 6 + (2 x 8) = 22 electrons.
Conclusion:
Correct option is '1'.
(IIT JEE 1980)
1) 2
2) 4
3) 6
4) 8
Logic & Solution:
In N2 molecule, there is a triple bond between two nitrogen atoms. Hence the number of electrons that participate in bond formation is 6. Each nitrogen atom contributes three electrons for bond formation.
Conclusion:
Correct option is '3'.
(IIT JEE 1993)
A) III & IV
B) I & II
C) I & III
D) II, III & IV
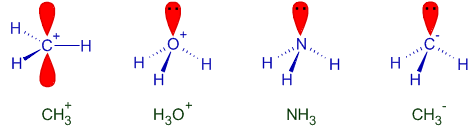
Logic & Solution:
| Species | (sum of atomic numbers of each atom) - charge on the species |
| I) CH3+ | C + 3H - (+1) = 6 + 3(1) - 1 = 8 |
| II) H3O+ | 3H + O - (+1) = 3(1) + 8 - 1 = 10 |
| III) NH3 | N + 3H - (0) = 7 + 3(1) - 0 = 10 |
| IV) CH3- | C + 3H - (-1) = 6 + 3(1) +1 = 10 |
H3O+, NH3 and CH3- are not only isoelectronic but are also iso-structural with pyramidal shape.
However CH3+ is trigonal planar with one empty orbital perpendicular to this plane.

Conclusion:
Correct option is 'D'.
| < Previous question | Next question > |
Author: Aditya vardhan Vutturi