
The alkyl halides are prepared from variety of sources like: alcohols, alkenes, alkanes etc.
Alcohols, R-OH can be converted to alkyl halides, R-X by using variety of reagents and reaction conditions.
Alkyl halides can be synthesized from alcohols by treating with hydrogen halides, HX (where X=Cl / Br / I). It is a nucleophilic substitution reaction.

* HCl and HBr can be liberated in-situ by using a combination of moderately concentrated sulfuric acid and a halide salt like NaCl, NaBr etc.
E.g.
![]()
However, 95% phosphoric acid is used instead of sulfuric acid to liberate HI from its salt.
* Among hydrohalic acids, HI is more reactive and HF is least reactive. Greater the H-X bond strength lesser is the reactivity. The order of reactivity of hydrogen halides with alcohols is: HF < HCl < HBr < HI
HCl requires a Lewis acid catalyst particularly with primary and secondary alcohols. HBr and HI can react without using any catalyst.
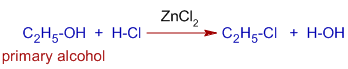
E.g. Conversion of ethyl alcohol, a primary alcohol to ethyl chloride requires ZnCl2, a Lewis acid.
* A combination of dry HCl gas and anhydrous ZnCl2 also known as "Lucas reagent" is employed to get alkyl chlorides. It is used to differentiate between primary, secondary and tertiary alcohols.
* The reactivity of various alcohols follows the order: methyl < primary < secondary < tertiary.
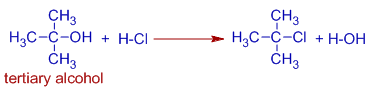
Indeed, tertiary alcohols react with HCl even in absence of a catalyst.
E.g. tert-butyl chloride can be synthesized by passing HCl gas into an aqueous solution of tert-butyl alcohol at low temperatures.
Reaction of alcohols with hydrogen halides is a nucleophilic substitution reaction. In general, the tertiary alcohols react by SN1 route, whereas primary and secondary alcohols react by SN2 mechanism .
In Unimolecular Nucleophilic Substitution (SN1), the rate of reaction is directly proportional to only the concentration of alcohol, R-OH. It does not depend on the concentration of hydrogen halide, HX.
rate ∝ [R-OH]
i.e. The rate determining step might have involved only one molecule of alcohol, R-OH.
E.g. The SN1 mechanism can be best described by reaction between tert-butyl alcohol and hydrochloric acid. This reaction occurs at faster rates at room temperature and its rate depends on the concentration of tert-butyl alcohol only. Rate is not affected by change in the concentration of hydrochloric acid.

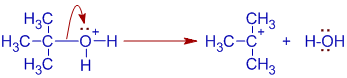
1) The initial step involves protonation of alcohol to give an alkaoxonium ion,.

2) Since the OH2+ is good leaving group, the oxonium ion immediately loses a water molecule to give a carbocation.
3) Finally, the halide ion attacks the carbocation to furnish alkyl halide.
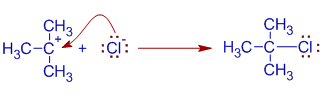
The formation of carbocation (step 2) is slow and is the rate determining step involving only one molecule/entity. Hence the mechanism is referred to as Unimolecular Nucleophilic Substitution, SN1.
It can be clearly seen from the following potential energy diagram for above mechanism.
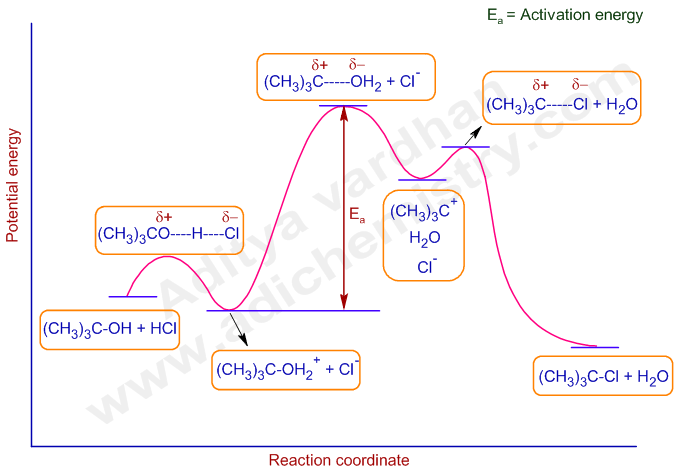
Look at the energy difference, Ea, between oxonium ion intermediate and the transition state that leads to carbocation. This is greater than the energy difference between any other intermediate and transition state. Hence the associated step must occur slowly than any other step and determines the rate of reaction. That is why the rate law involves only one molecule. i.e. Unimolecular Nucleophilic Substitution.
SN1 mechanism is shown by alcohols which can form carbocations easily.
As a consequence of Hammond's principle, the carbocations, which are more stable are also formed more easily. It is due to structural similarity between the transition state (that involves breaking of C-O bond) and carbocation intermediate formed from the transition state. The Hammond's postulate assumes that, during the course of a reaction, the nearest states with almost same energy are structurally similar and have comparable stability.
Since tertiary carbocation is more stable due to +I effect and also hyperconjugation effect shown by alkyl groups, the transition state leading to carbocation is also stabilized in the same way. Therefore, formation of carbocation is easier requiring less energy for the breaking of C-O bond.
Therefore we can restate that:
SN1 mechanism is shown by alcohols which can form stable carbocations.
The carbocation is planar with sp2 hybridized central carbon. Hence the attack of the nucleophile from either side is equally probable. Hence, if the starting alcohol is optically active due to chirality of -OH group bearing carbon, a racemic mixture (or sometimes a mixture of diastereomers) of alkyl halide is resulted.
E.g. When optically pure (3S)-3-methyl-3-hexanol is treated with a hydrogen chloride, a racemic mixture (50%:50% R, S) of 3-chloro-3-methylhexane is formed due to formation of carbocation via SN1 mechanism.
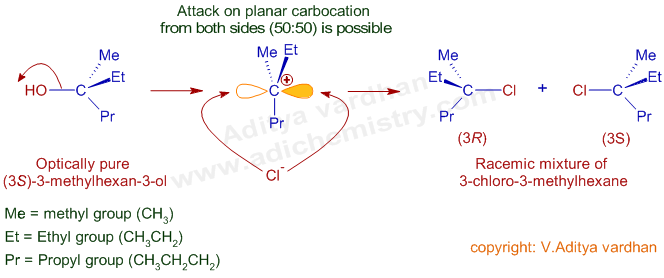
In this reaction, (3R)-3-chloro-3-methylhexane enantiomer is formed due to attack of Cl- ion from the left hand side leading to retention of configuration at chiral center. However, inversion of configuration occurs when the chloride ion attacks from the right hand side to yield (3S)-3-chloro-3-methylhexane enantiomer. Hence, a racemic mixture with 50:50 R and S alkyl halide is resulted.
Primary and secondary alcohols react by Bimolecular Nucleophilic substitution, SN2 mechanism, since the formation of primary and secondary carbocations is not easy and requires higher energy for breaking of C-O bond. Hence they are less stable and are not readily formed compared to tertiary carbocations.
Hence these alcohols adopt SN2 route in which the breaking of C-O bond and making of bond between carbon and nucleophilic halide ion occur simultaneously. i.e. Both alcohol and halide ion are participating in the crucial step of reaction.
Hence, in SN2 mechanism, the rate appears to depend on the concentration of both alcohol, R-OH and hydrogen halide.
rate ∝ [R-OH][HCl]
i.e. The rate determining step must involve two molecules.
E.g. The reaction of Ethyl alcohol with HBr is rather slow and involves SN2 mechanism as shown below.
* The alcohol is protonated in acidic medium to give ethyloxonium ion, which cannot break easily to give carbocation.
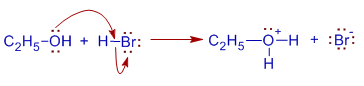
* Thus formed oxonium ion is attacked by the bromide ion from the back side while OH2+ group is still bonded to the carbon. It leads to a transition state in which the bromide ion facilitates the breaking of C-O bond. Both bond making and breaking occur simultaneously.
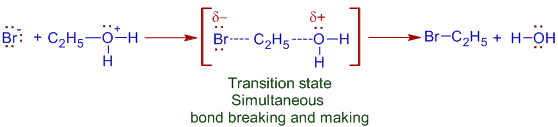
This is the slowest step and involves two chemical species i.e. molecularity is two. Hence this mechanism is referred to as Bimolecular Nucleophilic Substitution, SN2.
The SN2 mechanism involves inversion of configuration at chiral carbon, since the halide ion attacks the carbon (that is bonded to -OH group) from the opposite side of the C-O bond. Hence optically pure alkyl halide is obtained from optically pure alcohol due to complete inversion of configuration if the alcohol reacts by SN2 mechanism only.
For example, optically pure (2S)-butan-2-ol reacts by SN2 mechanism with HBr and gives (2R)-2-bromobutane as major product. The configuration at chiral carbon (that is bonded to alcoholic group) is inverted from (2S) to (2R) completely because only back side attack is possible in SN2 mechanism.
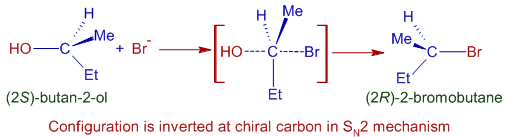
Note: However, some alkyl halide molecules with retention of configuration are also formed since some of the alcohol molecules may react by SN1 mechanism through carbocations.
| Alcohol | Reactivity with Lucas reagent |
| Primary (1o) | Turbidity appears after 10 minutes. Some times the reaction may take several days. |
| Secondary (2o) | In cold conditions, turbidity appears in 1-5 minutes. |
| Tertiary (3o) | Turbidity appears immediately in cold conditions. |
| Note: Appearance of turbidity is the indication of formation of alkyl halide. Turbidity appears since alkyl halides are less miscible with alcohols. They are formed as small droplets as in a suspension. | |