The physical properties of organohalogen compounds (alkyl halides and aryl halides) are influenced by factors like: i) polar nature of C-X bond, ii) molecular size (or indirectly the molecular weight), iii) type of halogen, iv) branching in the carbon chain etc.
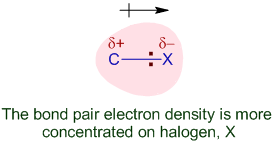
The bond between carbon and halogen atom (C-X) is polar. Since halogen atoms (except iodine) are more electronegative than carbon, there is partial accumulation of negative charge on halogen atom and positive charge on carbon. This is referred to as polarity and makes the molecule permanently dipolar.

The polar nature of C-X bond is expected to increase with increase in the electronegativity difference between carbon and halogen atom.
| Halogen | Electronegativity of halogen | Electronegativity difference between halogen and carbon |
| F | 4.0 | 4.0 - 2.5 = 1.5 |
| Cl | 3.5 | 3.5 - 2.5 = 1.0 |
| Br | 2.8 | 2.8 - 2.5 = 0.3 |
| I | 2.5 | 2.5 - 2.5 = 0 |
| Note: EN of carbon = 2.5 | ||
Hence the expected order of polarity of C-X bond will be: C-F > C-Cl > C-Br >> C-I.
Actually, based on EN difference, C-I bond should be considered as non-polar since both carbon and iodine atoms have same EN i.e. 2.50.
The bond length of C-X bond depends on the nature of halogen as well as the hybridization of carbon atom.
i) The bond length increases with increase in the size of halogen atom. The C-X bond weakens with increase in the bond length and decrease in the overlap of atomic orbitals.
order of bond length: C-F < C-Cl < C-Br < C-I
order of bond strength (bond energy): C-F > C-Cl > C-Br > C-I
ii) The sp3 C-X bonds of alkyl halides are longer than sp2 C-X bonds of vinyl and aryl halides. In the latter case, the C-X bond has partial double bond character that arises due to delocalization of π electrons. Hence the bond strength also increases with increase in double bond character.
| Halide | CH3CH2Cl | CH2=CH-Cl | C6H5-Cl |
| Bond energy (in kJ) | 339 | 368.2 | 405.8 |
Dipole moment, μ is defined as the product of magnitude of charge accumulated on either of pole on a polar bond and the distance between the two poles. Thus dipole moments of organohalogen compounds depend on not only the polarity of the bond and also on the bond length.
It is expected that organofluorides possess highest dipole moment values since the C-F bond is more polar. However, usually organochlorides possess greater dipole moment values. It is due to cumulative result of polarity and longer bond length of C-Cl compared to C-F.
E.g. The dipole moments of methyl halides vary as follows.
| Methyl halide | CH3F | CH3Cl | CH3Br | CH3I |
| Dipole moment, μ (in Debye) | 1.847 | 1.860 | 1.830 | 1.636 |
The boiling and melting points are affected by intermolecular forces of attractions like van der Waals dispersion forces and van der Waals dipole-dipole attractions.
The van der Waals dispersion forces, also known as London dispersion forces, arise due to temporary dipoles induced in the molecules. The strength of these forces depends on the size of molecule. Greater the size and number of electrons, greater is the polarization and hence greater is the attraction.
However, the dipole-dipole attractions are permanent. The magnitude of dipole-dipole interactions depend on the polarity of C-X bond.
Since organohalogen compounds are comparatively more polar (due to presence of polar C-X bond), the permanent dipole-dipole inter-molecular attractions also exist along with temporary dispersion forces. However, there are only London dispersion forces operating in their parent hydrocarbons. Therefore, the melting and boiling points (MPs & BPs) of these compounds are greater than the hydrocarbons of comparable molecular weights.
The MPs and BPs of alkyl and aryl halides increase with increase in the molecular size or molecular weight. The main reason is strengthening of dispersion forces rather than permanent dipole-dipole interactions.
It is clearly evident from the melting and boiling points of methyl halides. Even though the polarity of the C-X bond decreases from methyl fluoride to methyl iodide, the MP and BP trend upwards. This is due to increase in the size of molecule with increase in the size of halogen atom that results in strengthening of dispersion forces of attractions. i.e. The London forces outweigh dipole-dipole forces.
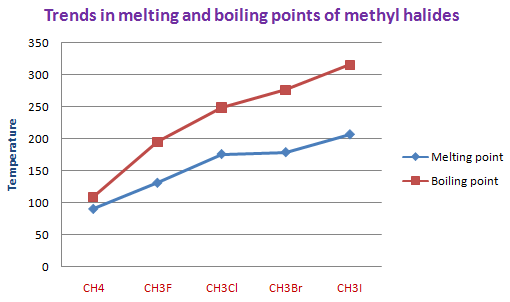
| Methyl halide | Molar mass (g mol-1) | Melting point | Boiling point | Physical state at room temperature |
| CH4 | 16.04 | 90.7 K (-182 oC) | 109-113 K (-160-164 oC) | Gas |
| CH3F | 34.03 | 131.4 K (-141.8 oC) | 195 K (-78.2 oC) | Gas |
| CH3Cl | 50.49 | 176 K (-97.4 oC) | 248.95 K (-24.2 oC) | Gas |
| CH3Br | 94.94 | 179 K (-93.66 oC) | 276.71 K (3.56 oC) | Gas |
| CH3I | 141.94 | 206.7 K (-66 oC) | 315.5-315.9 K (42-43 oC) | Liquid |
The melting & boiling points of alkyl halides are also affected by the size of and branching in alkyl chain. In a given homologous series, the MP and BP are increased from lower member to higher member.
E.g.
| CnH2n+1Cl | CH3Cl | CH3CH2Cl | CH3CH2CH2Cl | CH3CH2CH2CH2Cl |
| Melting point | 176 K (-97.4 oC) | 150 K (-122.8 oC) | 150.25 K (-122.9 oC) | 150 K (-123.1 oC) |
| Boiling point | 248.95 K (-24.2 oC) | 134 K (-139 oC) | 320 K (46.7 oC) | 351 K (78 oC) |
| Physical state at RT | Gas | Gas | Liquid | Liquid |
The boiling points decrease with increase in the branching of carbon chain. With branching, the surface area of contact between molecules decreases and hence strength of attractions between them also decreases. BPs are more affected by branching than MPs.
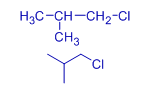
The melting points are also affected by symmetry of the molecule. The symmetrical molecules fit better into the crystal lattice and hence possess higher melting points. This is evident from the higher melting point of tert-butyl chloride. It is more spherical and hence fits better than other butyl chlorides into the lattice.
| Alkyl chloride | butyl chloride | sec-butyl chloride | Isobutyl chloride | tert-butyl chloride |
| Structure | 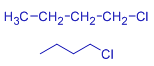 |
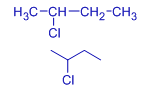 |
 |
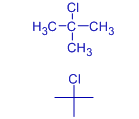 |
| Melting point | 150 K (-123.1 oC) | 141.85 K (-131.3 oC) | 142.85 K (-130.3 oC) | 247.55 K (-25.6 oC) |
| Boiling point | 351 K (78 oC) | 341.35 K (68.2 oC) | 341.65 K (68.5 oC) | 324.05 K (50.9 oC) |
| Physical state at RT | Liquid | Liquid | Liquid | Liquid |
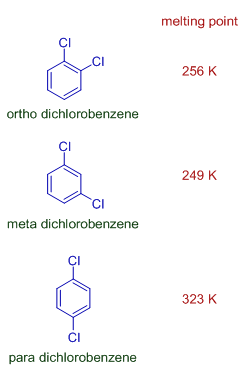
The para isomer of dihalo substituted benzenes is more symmetrical than corresponding ortho and meta isomers and hence possess higher melting point.
E.g. Para dichlorobenzene has higher melting point than ortho and meta dichlorobenzenes.
The densities of organohalogen compounds are related to mass as well as intermolecular attractive forces and tend to parallel boiling points. The density increases from fluorides to iodides.
In general, the density of monofluoro and monochloro alkanes are usually less than that of water, whereas monobromo and monoiodo alkanes are denser than water.
However poly halo compounds are denser than water.
Organohalogen compounds are sparingly soluble in water since they are comparatively less polar than water. They cannot mix with water molecules since breaking of stronger hydrogen bonds in water requires more energy and cannot be compensated by the attractions formed between water and organohalogen molecules. Less energy is released when new attractions are set up between the organo-halides and the water molecules as these are not as strong as the H- bonds in water.
However these compounds are miscible with non polar organic solvents because the new intermolecular attractions formed between molecules of organohalogens and solvent have much the same strength as the ones being broken.