
In IUPAC nomenclature, both substitutive and functional class systems are used while naming organohalogen compounds.
In the substitutive system, the halogen atom is not considered as functional group. It is regarded as a substituent and is denoted by the prefix: "halo" like "fluoro", "chloro", "bromo" or "iodo".
However, the halogen group is considered as a functional group in "common/trivial system" as well as in "functional class system of IUPAC" and hence it is indicated by the suffix: "halide". Functional class names constitute the names of organic groups (like alkyl, aryl, vinyl etc.) followed by the class name "fluoride", "chloride", "bromide", or "iodide".
In general, the substitutive system is more used and preferred over the functional class system for naming organohalogen compounds according to IUPAC rules.
* In substitutive system of IUPAC nomenclature, the alkyl halides are named as "Haloalkanes".
In this system, a root word is chosen based on the number of carbon atoms present in the parent chain and then the primary suffix-"ane" is added. Finally "halo" is prefixed to it.

* The position of halogen on the main chain is indicated by an appropriate locant (or number), which are decided according to "the rule of first point of difference" as stated below.
When series of locants containing the same number of terms are compared term by term, that series which contains the lowest number on the occasion of the first difference is preferred.
The purpose of this rule is to give lower numbers to the substituents in a less tedious way.
E.g.
In the following alkyl halide, the parent chain contains three carbon atoms and hence the root word is "prop".
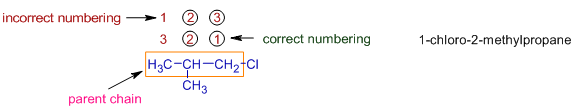
Since there is no unsaturation, the primary prefix is "ane".
There are two substituents i.e., chloro and methyl groups on the parent chain.
The counting should start from right most carbon, since the the series of locants: 1,2 is preferred over 2,3. In this way the substituents get least possible numbers. i.e. 1-chloro and 2-methyl.
The chloro group is written first in the name since it comes first according to the alphabetical order.
Thus the name of this compound is 1-chloro + 2-methyl + prop + ane = 1-chloro-2-methylpropane.
* Prefixes like di, tri, tetra are used when two or more same halogen atoms are present.
* When there are two different halogen atoms on different carbons on the parent chain, the numbering is done according to the rule of first point of difference. However, they are written in the alphabetical order in the name.
E.g. In the following molecule, the chloro group is given the least number, however, the bromo is written first in the name. Thus the IUPAC name is: 2-bromo-1-chloropropane.
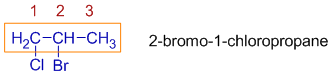
* If two halogen atoms are at equivalent positions, the one to be assigned the lower number is that cited first in the name.
E.g. In the following dihalide, the chloro and bromo groups are at equivalent positions. Since bromo comes first alphabetically, the counting is done from the right side. Hence the name is: 1-bromo-4-chlorobutane.
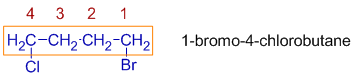
* However, the carbon with more substituents should be given least number as illustrated in the following example i.e. 4-bromo-1,1-dichlorobutane.

Since the weight of two chloro groups is more than one bromo, the counting is done from the left most carbon.
* The order of preference of halo group is same as groups like alkyl, nitro, ether etc. Hence above rules also apply when these substituents are present along with halo group.
E.g.
In 1-iodo-4-nitrobutane, the iodo group gets lower number since it has alphabetical preference.
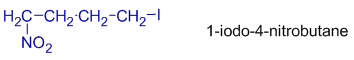
In 3-ethyl-4-fluorohexane, the ethyl group gets least number since 'e' comes first.
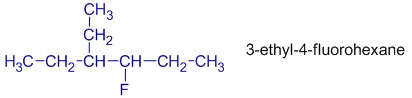
In "trivial system" or "function class system of IUPAC", these compounds are named as "alkyl halides". The name of alkyl group is derived from the name of parent hydrocarbon from which this group is obtained by removing a hydrogen atom.
Common and IUPAC names of some common alkyl halides are tabulated below.
| Structure of alkyl halide | Common name or Functional class IUPAC name Alkyl halide |
Substitutive IUPAC name Haloalkane |
Comments |
 |
methyl chloride | chloromethane | * The chlorine group is indicated by suffix chloride in common system and by prefix chloro in IUPAC system. * The root word is "meth", since there is only one carbon in the parent chain. |
 |
ethyl chloride | chloroethane | * The root word is "eth", since there are two carbons. * There is no need of indicating the position of chloro group. |
 |
propyl chloride | 1-Chloropropane | Now the position of chloro group must be indicated. Since this group is on the first position, it is denoted by 1. |
 |
Isopropyl chloride | 2-Chloropropane | * Chloro group is on the second carbon. |
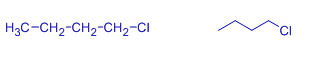 |
butyl chloride | 1-chlorobutane | |
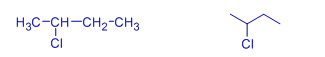 |
sec-butyl chloride | 2-chlorobutane | It is sec-butyl group since the chloro group is attached to secondary carbon and the carbon chain is linear. |
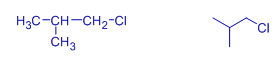 |
Isobutyl chloride | 1-chloro-2-methylpropane | * Now it is Isobutyl group, since it is branched and there is a methyl group on the second carbon. However, it is a primary alkyl halide. |
 |
tert-butyl chloride | 2-chloro-2-methylpropane | It is a tertiary alkyl halide. |
 |
Neopentyl chloride | 1-chloro-2,2-dimethylpropane | There are two methyl groups on the second carbon of the longest chain. These are denoted by "2,2-dimethyl". |
Note: In substitutive IUPAC system, the name of an organic compound is written as one word.
Some important di, tri and tetra halides and polyhalogenated compounds are:
| Structure | Common name | IUPAC name |
Comment |
| methylene chloride | dichloromethane | ||
| chloroform | trichloromethane | ||
| carbontetrachloride | tetrachloromethane | ||
 |
ethylidene dichloride | 1,1-dichloroethane | A gem dihalide |
 |
ethylene dichloride | 1,2-dichloroethane | A vicinal dihalide |
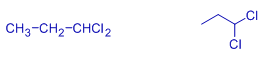 |
propylidene dichloride | 1,1-dichloropropane | |
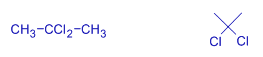 |
isopropylidene dichloride | 2,2-dichloropropane | |
 |
propylene dichloride | 1,2-dichloropropane | * The right most carbon is taken as first carbon. Always we should count from that side so as to get least numbers to the substituents. * If we start counting from left most carbon, then the substituents do not get least possible numbers. |
 |
Westron | 1,1,2,2-tetrachloroethane |
NOTE:
Geminal dihalides: contain two halogen atoms on same carbon atom.
Vicinal dihalides: contain two halogen atoms on adjacent carbon atoms.
* The double and triple bonds should be given lower numbers than halo groups, since they come first in the order of preference.
E.g.
![]()
In above compound (5-iodopent-2-ene), the counting starts from the left carbon since double bond must get least number than iodo group.
Some important unsaturated organohalogen compounds are shown below.
| Structure | Common name | IUPAC name | Comment |
 |
vinyl chloride | chloroethene | The chlorine atom is attached to sp2 carbon |
 |
allyl chloride | 3-chloroprop-1-ene | The halo group is attached to sp3 carbon |
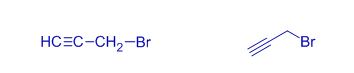 |
propargyl bromide | 3-bromoprop-1-yne | |
 |
Westrosol | 1,1,2-trichloroethene | It is an industrial solvent |
The organohalogen compounds containing cyclic moieties, the infix: "cyclo" is added immediately before the root word.
E.g.
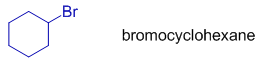
Notice the infix: cyclo between hexane and bromo in bromocyclohexane.
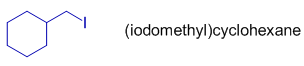
In above molecule, the iodo group is present on CH2. Hence the whole group is denoted as "iodomethyl".

In above compound, the double bond is given least number as usual. But there is not need to indicate its position in the name.
The organohalogen compounds containing halogen atoms attached directly to aromatic rings like benzene are called aryl halides or haloarenes. When the aromatic ring is benzene they are named as halobenzenes.
E.g.
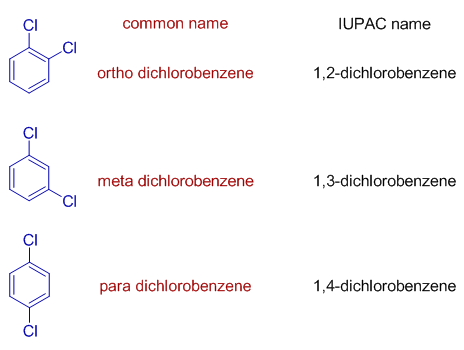
In common system, the isomers of disubstituted benzenes are indicated by terms: ortho, meta and para, when the two substituents are on 1,2 ; 1,3 and 1,4 positions respectively.
In the following dihalo substituted benzene, 1-bromo-4-chlorobenzene, numbering starts from bromine.
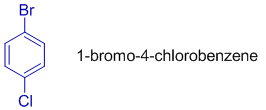
In 1-ethyl-4-fluorobenzene, ethyl group has more preference and given least number.

In 1-bromo-4-fluoro-2-iodobenzene, counting starts at bromine and going anti clockwise to fluorine through iodine, since this way of counting gives lowest possible numbers (1,2,4) to the substituents. Notice that fluoro is written first in the name before iodo due to alphabetical order.
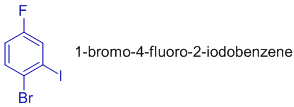
Also see the order of writing halo groups in the name of following compound, 2-bromo-4-fluoro-1-iodobenzene.

Again ethyl group takes preference over other groups i.e. fluoro and methoxy due to alphabetical reason in the following compound, 1-ethyl-3-fluoro-5-methoxybenzene.

Finally, the IUPAC name of benzyl chloride is (chloromethyl)benzene. The CH2Cl group is called chloromethyl group.

| < Introduction: organohalogen compounds | Organo halogen compounds | Physical properties of organohalogens> |