SYSTEMATIC IUPAC NAME FORMAT
The systematic IUPAC name of an organic compound consists of four parts.

The suffix is again divided into primary and secondary. Therefore, the complete systematic IUPAC name can be represented as:
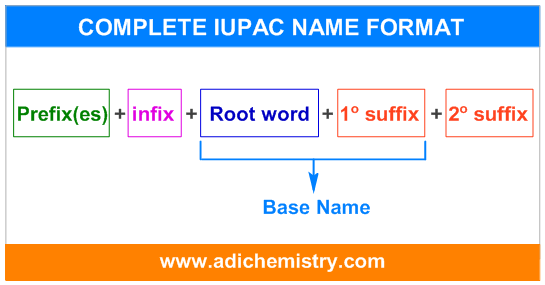
* The "word root" and "1o suffix" together is known as base name.
* The Prefix(es), infix and 2o suffix may or may not be required always.
1) Root word:
The Word root of IUPAC name indicates the number of carbon atoms in the longest possible continuous carbon chain also known as parent chain chosen by a set of rules. The word roots used for different length of carbon chain (upto 20) are shown below.
| Number of carbon atoms in the parent chain | Root word |
| 1 | Meth |
| 2 | Eth |
| 3 | Prop |
| 4 | But |
| 5 | Pent |
| 6 | Hex |
| 7 | Hept |
| 8 | Oct |
| 9 | Non |
| 10 | Dec |
| 11 | Undec |
| 12 | Dodec |
| 13 | Tridec |
| 14 | Tetradec |
| 15 | Pentadec |
| 16 | Hexadec |
| 17 | Heptadec |
| 18 | Octadec |
| 19 | Nonadec |
| 20 | Icos |
2) Suffix:
It is again divided into two types.
- Primary suffix and
- Secondary suffix
i) Primary suffix:
It is used to indicate the degree of saturation or unsaturation in the main chain. It is added immediately after the word root of IUPAC name.
| Type of carbon chain | Primary suffix |
| Saturated (all C-C bonds) | -ane |
| Unsaturated: one C=C | -ene |
| Unsaturated: two C=C | -diene |
| Unsaturated: one C≡C | -yne |
| Unsaturated: two C≡C | -diyne |
| Unsaturated: one C=C & one C≡C | -enyne |
ii) Secondary suffix:
It is used to indicate the main functional group in the organic compound and is added immediately after the 1o suffix in the IUPAC name.
Note: If there are two or more functional groups in a compound, the functional group with higher priority is to be selected as main functional group, which must be indicated by a secondary suffix. The remaining functional groups with lower priority are treated as substituents and are indicated by prefixes.
The suffixes as well as prefixes used for some important functional groups are shown in the following table in the decreasing order of their priority.
Also note that different suffix is used when carbon atom of the functional group is not part of the main chain.
| Name of Functional group | Representation | Suffix
When carbon of the functional group is part of the parent chain |
Suffix
When carbon of the functional group is NOT part of the parent chain |
Prefix |
| carboxylic acid | -COOH | -oic acid | -carboxylic acid | carboxy- |
| Acid anhydride | -oic anyhydride | -carboxylic anhydride | - | |
| Ester | -COOR | alkyl -oate | alkyl -carboxylate | alkoxycarbonyl- |
| Acid halide | -COX | -oyl halide | -carbonyl halide | halocarbonyl- |
| Acid amide | -CONH2 | -amide | -carboxamide | carbamoyl- |
| Nitrile | -CN | -nitrile | -carbonitrile | cyano- |
| Aldehyde | -CHO | -al | -carbaldehyde | oxo- |
| Ketone | -CO- | -one | - | oxo- |
| Alcohol | -OH | -ol | - | hydroxy |
| Thiol | -SH | -thiol | - | mercapto |
| Amine | -NH2 | -amine | - | amino- |
| Imine | =NH | -imine | - | imino- |
| Alkene | C=C | -ene | - | - |
| Alkyne | C≡C | -yne | - | - |
Note: This is not the complete reference.
3) Prefix:
The prefix is used to indicate the side chains, substituents and low priority functional groups (which are considered as substituents). The prefix may precede the word root or the infix of IUPAC name.
The prefixes used for some common side chains and substituents are shown below. (the prefixes for functional groups are already given)
| Side chain or Substituent | Prefix |
| -CH3 | methyl- |
| -CH2CH3 (or) -C2H5 | ethyl- |
| -CH2CH2CH3 | propyl- |
| isopropyl- | |
| -CH2CH2CH2CH3 | butyl |
| sec-butyl
(or) (1-methyl)propyl |
|
| isobutyl
(or) (2-methyl)propyl |
|
| tert-butyl
(or) (1,1-dimethyl)ethyl |
|
| -X | halo- |
| -OR | alkoxy- |
| -NO2 | -nitro |
Remember that the alkyl groups along with halo, nitro and alkoxy have the same preference. They have lower priority than double and triple bonds.
3) Infix:
The infixes, like cyclo, spiro, bicyclo, are added between the prefix(es) and root word in the IUPAC name to indicate the nature of parent chain.
* The "Cyclo" infix is used to indicate the cyclic nature of the parent chain.
* The "Spiro" infix is used to indicate the spiro compound.
* The "Bicyclo" infix is used to indicate the bicyclic nature of the parent chain.
The infixes are some times called as primary prefixes.
STEPS INVOLVED IN WRITING IUPAC NAME
1) The first step in giving IUPAC name to an organic compound is to select the parent chain and assign a word root.
2) Next, the appropriate primary suffix(es) must be added to the root word to indicate the saturation or unsaturation.
3) If the molecule contains functional group or groups, a secondary suffix must be added to indicate the main functional group. This is optional and not necessary if the molecule contains no functional group.
4) Prefix the root word with the infix "cyclo" if the parent chain is cyclic; or with the infix "spiro" if it is a spiro compound; or with the infix "bicyclo" if the compound is bicyclic.
5) Finally add prefix(es) to the IUPAC name, if there are side chains or substituents on the parent chain.
E.g. The IUPAC name of the following compound (3-methylbutan-2-ol) is arrived in steps mentioned below.
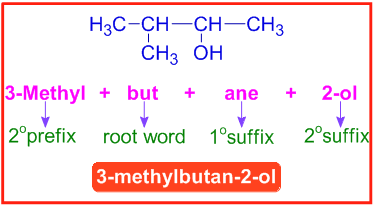
| Step-1 | How many carbons are there in the parent chain? | 4 | Root word = "but" |
| Step-2 | Saturated or Unsaturated? | Saturated | 1osuffix = "ane" |
| Step-3 | Is there any functional group? | Yes. There is an alcohol group on 2nd carbon. | 2osuffix = "2-ol" |
| Step-4 | Are there any side chains or substituents? | Yes. There is a methyl group on 3rd carbon. | 2oprefix = "3-methyl" |
Now add them to makeup the IUPAC name of the compound.
You will learn how to select a parent chain?; how to number the carbon atoms and give the locants to the functional groups, side chains ? etc., in the following section.
RULES OF IUPAC NOMENCLATURE
The following IUPAC nomenclature rules are helpful in assigning the systematic IUPAC name of an organic compound.
1) The selection of parent chain:
The first step in naming an organic compound is to select the parent chain and give the root word based on the number of carbon atoms in it.
The parent chain in an organic molecule is the longest continuous carbon chain containing as many functional groups, double bonds, triple bonds, side chains and substituents as possible.
Examples:
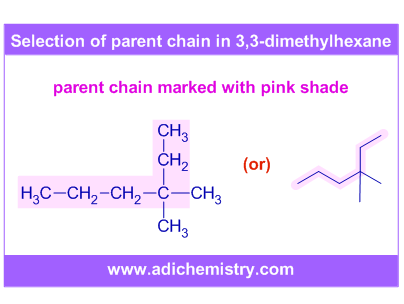
i) In the following molecule, the longest chain has 6 carbons. Hence the word root is "hex-". Note that the parent chain may not be straight.
ii) The root word for the following molecule is "hept-" since the longest chain contains 7 carbons.
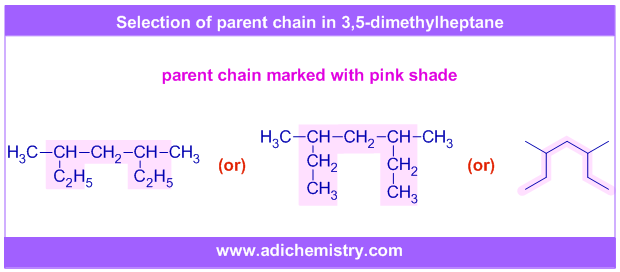
Do not come under the impression that the ethyl groups (-C2H5) are side chains and the longest chain contains 5 carbons.
The shaded part shows the longest chain that contains 7 carbons. Also look at the alternate way of writing this molecule in which the ethyl groups are expanded to -CH2CH3.
iii) In the following molecule, there are three chains of equal length (7 carbons).
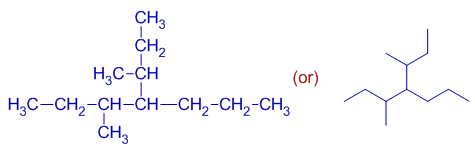
However, the chain with more number of substituents (that with 3 substituents as shown in the following diagram) is to be taken as the parent chain. Thus "hept" appears as word root in the IUPAC name of this compound.
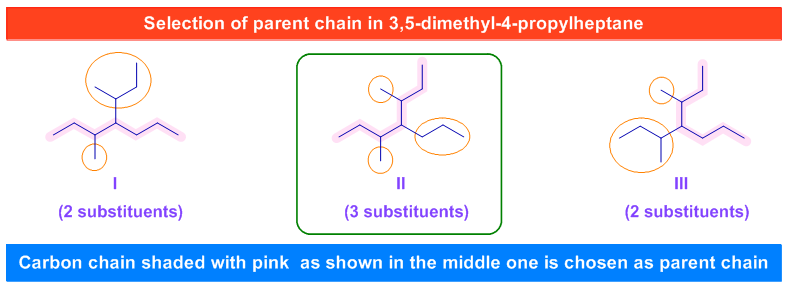
iv) The double bonds and triple bonds have more priority than the alkyl side chains and some other substituents like halo, nitro, alkoxy etc. Hence, whenever there are two or more chains with equal number of carbons, the chain that contains double or triple bond is to be selected as the parent chain irrespective of other chain containing more number of substituents.
hex-1-ene.png)
There are two chains with 6 carbons. But the chain with the a double bond as shown in the diagram (II) is to be selected as the parent chain.
hex-1-ene.png)
Note: The double bond has more priority than the triple bond.
v) However, the longest chain must be selected as parent chain irrespective of whether it contains multiple bonds or not.
E.g. In the following molecule, the longest chain (shaded) contains no double bond. It is to be selected as parent chain since it contains more carbons (7) than that containing double bond (only 6 carbons).
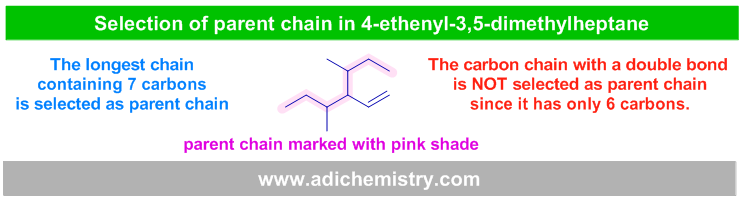
vi) The chain with main functional group must be selected as parent chain even though it contains less number of carbons than any other chain without the main functional group.
The functional group overrides all of above rules since it has more priority than the double bonds, triple bonds, side chains and other substituents.
Remember that the functional group is king.
E.g. The chain (shaded) with 6 carbons that includes the -OH functional group is to be selected as parent chain irrespective of presence of another chain with 7 carbons that contains no functional group.
-4-methylhexan-2-ol.png)
There are other situations which will decide the parent chain. These will be dealt at appropriate sections.
2) Numbering the parent chain:
i) The positions of double bonds or triple bonds or substituents or side chains or functional groups on the parent chain are to be indicated by appropriate numbers (or locants). The locants are assigned to them by numbering carbon atoms in the parent chain.
Even though two different series of locants are possible by numbering the carbon chain from either sides, the correct series is chosen by following the rule of first point of difference as stated below.
Note: In iupac nomenclature, the number which indicates the position of the substituent is called 'locant'.
The rule of first point of difference:
When series of locants containing the same number of terms are compared term by term, that series which contains the lowest number on the occasion of the first difference is preferred.
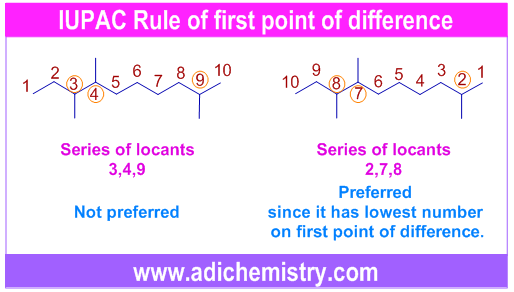
For example, in the following molecule, the numbering can be done from either side of the chain to get two sets of locants. However the 2,7,8 is chosen since it has lowest number i.e., 2 on the first occasion of difference when compared with the other set: 3,4,9.
Actually the so called “Least Sum Rule” is the special case of above “Rule of First point of Difference”. Though looking simple, the least sum rule is valid only to chains with two substituents, a special case. However use of Least sum rule is not advisable when there are more than two substituents since it may violate the actual rule of first point of difference.
Therefore, while deciding the positions, we should always use "the rule of first point of difference" only.
ii) If two or more side chains are at equivalent positions, the one to be assigned the lower number is that cited first in the name.
In case of simple radicals, the group to be cited first in the name is decided by the alphabetical order of the first letter in case of simple radicals. While choosing the alphabetical order, the prefixes like di, tri, tetra must not be taken into account.
In the following molecule, 4-ethyl-5-methyloctane, both methyl and ethyl groups are at equivalent positions. However the ethyl group comes first in the alphabetical order. Therefore it is to be written first in the name and to be given the lowest number.
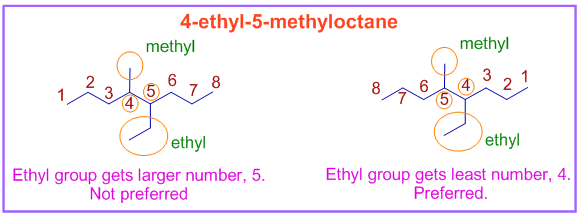
Note: The groups: sec-butyl and tert-butyl are alphabetized under "b". However the Isobutyl and Isopropyl groups are alphabetized under "i" and not under "b" or "p".
iii) However, if two or more groups are not at equivalent positions, the group that comes first alphabetically may not get the least number.
E.g. In the following molecule, 5-ethyl-2-methylheptane, the methyl and ethyl groups are not at equivalent positions. The methyl group is given the least number according to the rule of first point of difference.
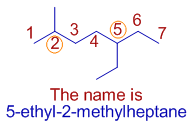
But note that the ethyl group is written first in the name.
iv) The multiple bonds (double or triple bonds) have higher priority over alkyl or halo or nitro or alkoxy groups, and hence should be given lower numbers.
E.g. In the following hydrocarbon, 6-methylhept-3-ene, the double bond is given the lower number and is indicated by the primary suffix 3-ene. The position of methyl group is indicated by locant, 6.
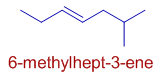
v) The double bond is preferred over the triple bond since it is to be cited first in the name.
Therefore the double bond is to be given the lower number whenever both double bond and triple bond are at equivalent positions on the parent chain.
E.g. In the following hydrocarbon, hept-2-en-5-yne, both the double and triple bonds are at equivalent positions. But the position of double bond is shown by 2-ene. The counting of carbons is done from the left hand side of the molecule.
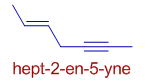
vi) However, if the double and triple bonds are not at equivalent positions, then the positions are decided by the rule of first point of difference.
E.g. In the following hydrocarbon, hept-4-en-2-yne, the double and triple bonds are not at equivalent positions. The triple bond gets the lower number.
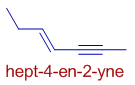
Again note that the 4-ene is written first.
vii) Nevertheless, the main functional group must be given the least number even though it violates the rule of first point of difference. It has more priority over multiple bonds also.
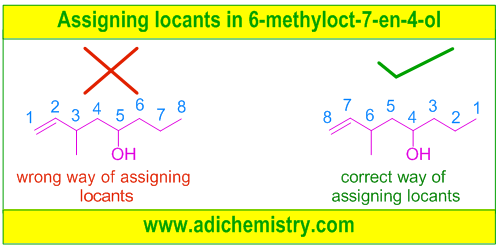
For example, in the following organic molecule, 6-methyloct-7-en-4-ol, the -OH group gets lower number (i.e., 4) by numbering the carbons from right to left.
3) Grammar to be followed in writing the IUPAC name:
i) The IUPAC name must be written as one word. However, there are exceptions.
ii) The numbers are separated by commas.
iii) The numbers and letters are separated by hyphens.
iv) If there are two or more same type of simple substituents they should be prefixed by di, tri, tetra, penta etc.
E.g. The number of methyl groups are indicated by di and tri in the following cases.

v) If the side chains themselves contain terms like di, tri, tetra etc., the multiplying prefixes like bis, tris, tetrakis etc., should be used.
E.g. The two 1,2-dimethylpropyl groups are indicated by the prefix "bis" as shown below.
nonane.png)
vi) If two or more side chains of different nature are present, they are cited in alphabetical order.
* In case of simple radicals, they are alphabetized based on the first letter in the name of simple radical without multiplying prefixes.
E.g. In the following molecule, the ethyl group is written first since the letter 'e' precedes the letter 'm' of methyl in the alphabetical order. We should not compare 'e' in the word 'ethyl' and 'd' in the word 'dimethyl'
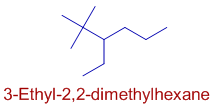
* However the name of a complex radical is considered to begin with the first letter of its complete name.
E.g. In the following case, “dimethylbutyl” is considered as a complete single substituent and is alphabetized under "d".
nonane.png)
IUPAC Nomenclature of cyclic compounds
i) The IUPAC name of an alicyclic compound is prefixed with "cyclo".
E.g.

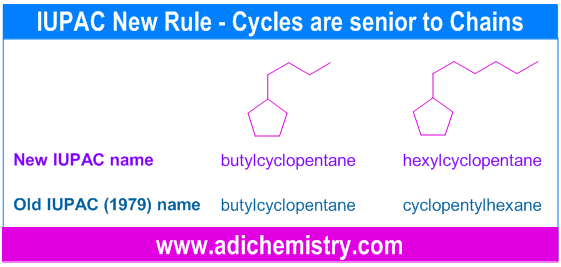
ii) Cycles are seniors to acyclics.
Hence when cyclic nucleus is attached to the non cyclic chain, it is always named as the derivative of the cyclic hydrocarbon irrespective of the length of the non cyclic chain. This is a very new IUPAC recommendation.
However, according to the 1979 convention: “a hydrocarbon containing a small cyclic nucleus attached to a long chain is generally named as a derivative of the acyclic hydrocarbon; and a hydrocarbon containing a small group attached to a large cyclic nucleus is generally named as a derivative of the cyclic hydrocarbon.” Most of the textbooks and teachers still follow this convention.
E.g. In the following examples, the old IUPAC system suggests different name when the acyclic chain contains more number of carbons than in cyclic system.
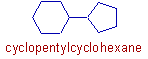
iii) When two non-aromatic rings (alicyclic) are connected to each other, the compound is considered as the derivative of larger ring. The root word is derived from the larger ring. Whereas the smaller ring is indicated by the prefix.
E.g. The following compound is considered as the derivative of cyclohexane. The smaller ring is indicated by the prefix: cyclopentyl.
iv) However if two alicyclic rings of same size are connected to each other, they are named as x,x'-bi(cycloalkyl). Where x and x' indicate the locants given to carbons through which the rings are connected. The x refers to the locant of carbon in first ring and x' represents the locant of carbon in second ring.
E.g. The following compound is named as 1,1'-bi(cyclopentyl) since there are two cyclopentane rings are connected to each other through their 1 and 1' carbons.
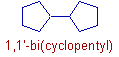
E.g. In the following compound two cyclopentane rings are attached to each other. Hence the name is 1.1'-bi(cyclopentyl)
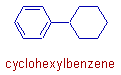
v) The aromatic rings have more preference over the non-aromatic rings, when the sizes of both the rings are same.
E.g. The word root is benzene in the following compound.
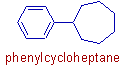
However the larger ring has more priority irrespective of its nature (whether it is aromatic or not).
E.g. In the phenylcycloheptane, the non-aromatic ring, cycloheptane is larger. Hence this compound is named as the derivative of cycloheptane.
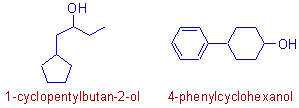
vi) Nevertheless, the functional group is always the king. It will decide the root word of the IUPAC name when present in the compound.
E.g. In the first compound as shown below, the acyclic chain is taken as parent chain since it has the -OH functional group on it. The cyclopentane part is considered as substituent.
In the second compound also the benzene ring is considered as substituent since it contains no functional group.
Find more examples on iupac names of cyclic compounds.
IUPAC name of Compounds with multi functional groups
Whenever there are more than one functions group, the main functional group is indicated by the 2o suffix in the IUPAC name, whereas the remaining functional groups are considered as substituents and are indicated by the appropriate prefixes.
E.g. In the following organic compound, 5-hydroxyhexanoic acid, both -OH and -COOH groups are the functional groups. But the -COOH group has more priority than the -OH group. Hence it is considered as the main functional group and indicated by secondary suffix, "oic acid". Whereas the -OH group is considered as substituent and is indicated by the prefix, "hydroxy".
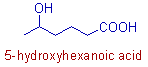
Jump to next page - more IUPAC examples.
IUPAC nomenclature of Spiro compounds
The spiro compounds contain two cyclic rings that share one common carbon atom, which is called as the spiroatom.
The IUPAC name of spiro compound has the infix "spiro" followed by square brackets inside of which the number of atoms in the smaller ring followed by the number of atoms in the larger ring, excluding the spiroatom itself, are shown. These numbers are separated by a period (dot).
The word root of the compound is based on the total number of skeletal carbons in the two cycles including the spiroatom. Do not include the carbons of side chains and substituents over the rings while counting this number.
E.g. In the following spiro compound, there is one carbon atom common to 5 membered and 6 membered rings. The IUPAC name is spiro[4.5]decane. Notice that the spirocarbon is not taken into account while giving the numbers in the square bracket.
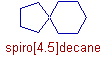
The numbering is done starting from skeletal carbon of small ring and continued until the spiro carbon. Then the skeletal carbons in the larger ring are numbered.
E.g. In the following spiro compound the methyl group has got the locant, 7. It is because the numbering of the spiro skeleton is done first and it is not necessary that the methyl group should get the least number always.
![7-methylspiro[4.5]decane](iupac1-25a.gif)
More IUPAC examples of spiro compounds on the next page.
IUPAC nomenclature of Fused bicyclic compounds
The bicyclo compounds contain two fused rings with two connecting common carbon atoms known as bridge head carbons. The carbon chain or covalent bond connecting these bridge heads is considered as a bridge. There are three bridges in a simple bicyclic compound.
The IUPAC name of bicyclic compound has the infix "bicyclo" followed by square brackets showing the numbers separated by periods (dots). They indicate the number of atoms in the bridges. While counting the number of atoms in the bridge, the bridge head carbons are not counted. These numbers are arranged in the decreasing order i.e., from larger bridge to smaller one.
The root word indicates the total number of skeletal carbon atoms in the two rings. Do not include the carbons in side chains or substituents over the rings while arriving at the word root of IUPAC name.
E.g. In the following bicyclo compound, there are three bridges with 2, 2 and 1 carbon atoms connecting the two bridge head carbons. Hence the name is bicyclo[2.2.1]heptane.
![bicyclo[2.2.1]heptane](iupac1-26.gif)
The numbering is done starting from one of the bridge head carbon and continued through the longest bridge until another bridge is reached. Then the skeletal carbons of next longer bridge are numbered. This process is continued until the shortest bridge in finally numbered.
E.g. In the following bicyclo compound, the methyl group is is considered to be at 7th position.
![7-methylbicyclo[2.2.1]heptane](iupac1-26a.gif)
Jump to more examples iupac nomenclature of bicyclo compouns.