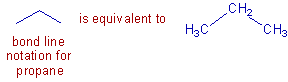
| < How to write the structural formulae of organic compounds | Organic chemistry: Home page | Organic IUPAC nomenclature |
The structural formulae of organic molecules can also be represented by bond line notation method. In this method, the carbon and hydrogen atoms are not shown, but only the bonds between the carbon atoms are shown as lines. The ends and vertices represent the carbon atoms. The number of hydrogens must be guessed according to the valence rules. However, atoms other than carbon and hydrogen are shown.
For example, the propane molecule can be represented as:
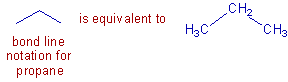
In the above diagram, the vertex and the ends of the lines represent the carbon atoms. The middle carbon contains only two hydrogens since it has already formed two bonds with other carbons.
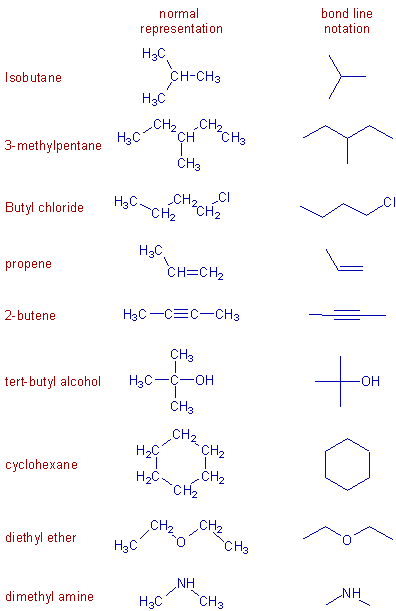
Some more examples are shown below.
Author: Aditya vardhan Vutturi