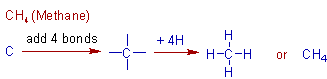
| < Introduction to basic concepts in organic chemistry | Organic chemistry: Home page | Bond line notation > |
Already we have seen the bonding nature of carbon in the previous section. Now let us familiarize ourselves with the structural formulae of some organic molecules. On this page, we will build them up with variety of combinations of different atoms using valence rules. While doing so we should keep in our mind that it is customary that every organic molecule must contain at least one carbon atom.
The organic compounds are categorized into various types based on the functional group or groups present. The simplest of all are saturated acyclic hydrocarbons otherwise known as alkanes. It is possible to get most of the organic compounds by modifying these alkanes.
Now let us write the structural formulae of alkanes.
Before writing the formulae of alkanes, it is necessary to understand the meaning of homologous series.
A Homologous series refers to organic compounds with a similar general formula and possessing similar chemical properties due to presence of same functional group and exhibiting a regular gradation of physical properties with increase in molecular weight.
The difference between the formulae of two successive compounds in a homologous series is CH2.
Alkanes constitute homologous series of saturated acyclic hydrocarbons represented by the general formula CnH2n+2.
Where n = number of carbon atoms.
The hydrocarbons are organic compounds containing only carbon and hydrogen as constituent atoms.
The word saturated indicates the presence of only single bonds between two carbon atoms, C-C.
That means, in alkanes, all the carbon atoms must be sp3 hybridized and form four single bonds with other atoms. In this case other atoms refer to carbon and hydrogen only.
Construction of CH4 (methane) molecule:
Let us start with the first alkane containing one carbon (n=1). Substitution of this value in the general formula, CnH2n+2 gives CH4. It is named as methane.
It can be constructed by connecting a carbon with four hydrogen atoms. Remember that the carbon is tetravalent. The stepwise construction of methane molecule is shown below.
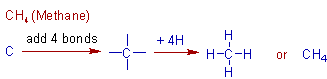
Construction of C2H6 (ethane) molecule:
The second alkane contains two carbons and its formula is C2H6. It is named as ethane. There is a C-C bond in this molecule. The stepwise construction of ethane molecule is shown below.
Remember that each carbon in any given alkane forms only four single bonds.
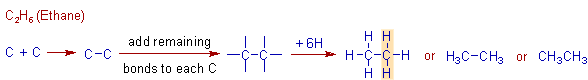
Also note that the shaded area, which shows how the ethane molecule differs by a 'CH2' group from methane molecule. We can also think that the ethane molecule is obtained by inserting a CH2 group in between a C and H of methane.
Construction of C3H8 (propane) molecule:
Now let us move on to the third alkane named as propane containing three carbons. Its formula is C3H8. The three carbon atoms are linked to each other linearly and the 8 hydrogens are attached to them as shown below.
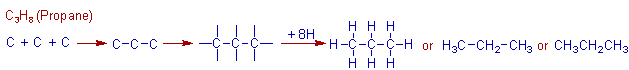
Construction of butane & isobutane molecules:
The next formula containing four carbon atoms is C4H10. However the 4 carbon atoms can be arranged in a linear chain or in branched manner. Therefore two structurally different alkanes containing 4 carbons are obtained as shown below. These molecules with linear and branched chains are named as butane and isobutane respectively. They possess same molecular formula but differ in structures and hence are known as structural isomers.
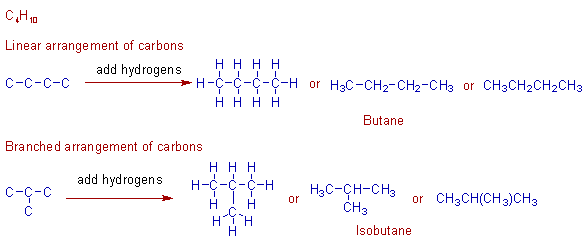
It is very important to note that all of the following structures are one and same and indicate the different ways of writing the structure of isobutane.
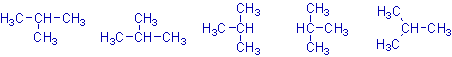
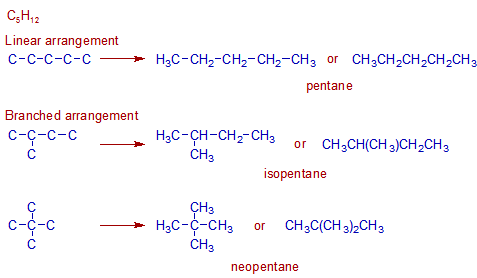
Construction of isomers of C5H12:
There are three structural isomers possible with the formula C5H12 as shown below.
It is also possible to write the higher members of alkanes using the same principles applied as above.
Note: You need not worry about the names of these organic compounds now. We will learn the naming of organic compounds in next sections.
H.W: Write the structural formulae of different isomers of C6H14.
Alkenes constitute the homologous series of unsaturated acyclic hydrocarbons containing a carbon-carbon double bond and are represented by the general formula CnH2n.
There must be one and only one double bond between two carbon atoms. That means there are two carbon atoms undergoing sp2 hybridization and the remaining are sp3 hybridized.
Every sp2 hybrid carbon forms a double bond with another carbon atom. They also form single bonds with either hydrogens or with carbons.
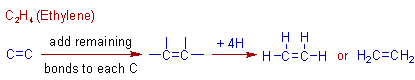
Construction of C2H4 (ethylene) molecule:
The first alkene contains two carbons since a C=C is must. Its formula is C2H4. It is named as ethylene. The construction of ethylene molecule is shown below.
Construction of C3H6 (propylene) molecule:
The second alkene in the series is propylene containing three carbons. There are one C=C and one C-C bonds in this molecule.
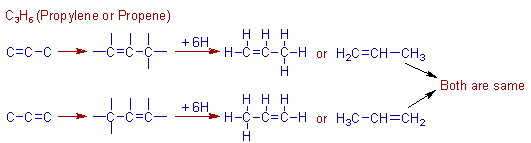
Construction of isomers of C4H8:
There are three isomers possible with C4H8 formula. i.e., 1-butene, 2-butene and isobutylene. These can be constructed as shown below.
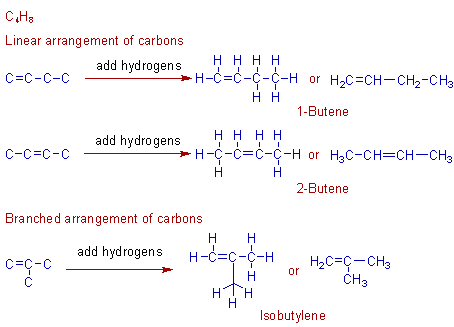
Both 1-butene and 2-butene contain linear arrangement of carbons. But they differ by the position of double bond. Hence these are called as positional isomers. However, still two more geometrical isomers are possible with 2-butene which will be dealt separately.
Isobutylene has branched carbon chain.
Alkynes constitute the homologous series of unsaturated acyclic hydrocarbons containing a carbon-carbon triple bond. The general formula is CnH2n-2.
The alkyne molecule contains only one triple bond between two carbon atoms. Only two carbon atoms are 'sp' hybridized and the remaining are sp3 hybridized.
Two 'sp' hybridized carbons form a triple bond with each other. These carbons also form one single bond with either hydrogens or carbons.
Construction of C2H2 (acetylene) molecule:
Again the first alkyne contains two carbons since there must be a C≡C. Its formula is C2H2. Its name is acetylene, which can be constructed as shown below:
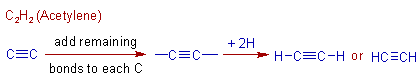
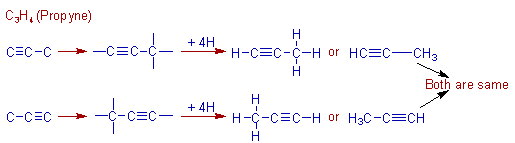
Construction of C3H4 (propyne) molecule:
The propyne molecule contains three carbons. There are one C≡C and one C-C bonds in this molecule.
Construction of isomers of C4H6:
There are only two isomers possible with C4H6 formula. i.e., 1-butyne and 2-butyne. These can be constructed as shown below.
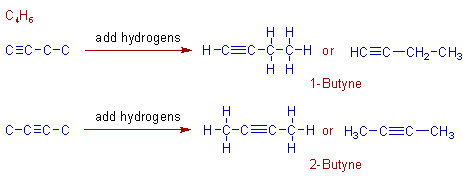
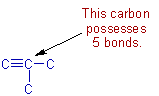
However the branched isomer is not possible since no carbon can form more than 4 bonds.
The alkyl halides are obtained by replacing the hydrogens on the alkane molecule. They are represented by CnH2n+1X (where X = any halogen).
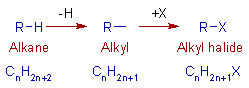
Types of carbons and hydrogens: It is necessary to know the types of carbons and hydrogens before constructing the alkyl halide molecules.
a) The primary (1o) carbon is the carbon that is linked directly to only one carbon in a molecule. The hydrogens attached to primary carbons are termed as primary hydrogens.
b) The secondary (2o) carbon is the carbon that is attached to two carbon atoms in a molecule. The hydrogens attached to secondary carbons are termed as secondary hydrogens.
c) The tertiary (3o) carbon is the carbon that is bonded directly to three carbons. The hydrogens attached to tertiary carbons are termed as tertiary hydrogens.
These are shown in the following diagram
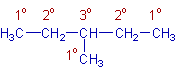
Construction of some of the alkyl halides are shown below along with their common names. However note that the names given may not be the systematic names coined by IUPAC nomenclature, which we will learn in next chapter.
The methyl group is obtained by removing one of the hydrogen atom from methane.
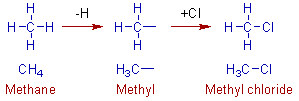
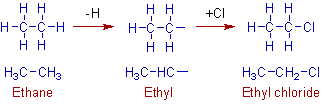
The ethyl group is obtained from ethane by removing a hydrogen atom from any of the carbon. In this case both the carbons are 1o. The removal of hydrogen from either of the carbons will result in the same ethyl group.
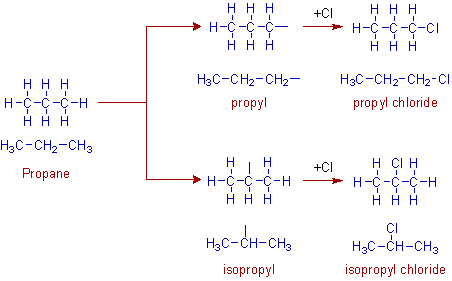
The removal of hydrogens from propane can be done in two ways:
i) One hydrogen atom from any of the end carbon (1o carbon) can be removed to get the propyl group.
ii) One hydrogen from the middle carbon (2o carbon) can be removed to get the isopropyl group.
Therefore two isomeric halides are possible from propane as shown below.
The removal of hydrogens from butane can also be done in two ways; i.e., either from 1o carbon or from 2o carbon. This results in two types of groups i.e., butyl and sec-butyl groups. The resultant alkyl halides are shown below.
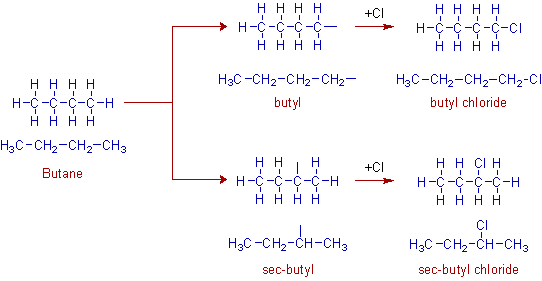
Note: Do not confuse. You might have expected the sec-butyl group is to be named as isobutyl. But this group is named as sec-butyl since the hydrogen is removed from secondary carbon.
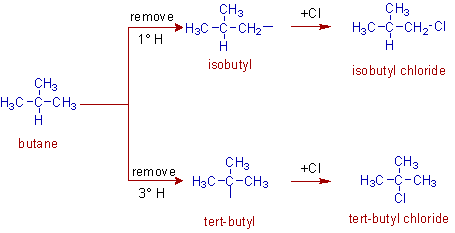
It is also possible to get two types of groups from isobutane by removing hydrogens either from 1o or 3o carbons to give isobutyl and tert-butyl groups respectively.
We will learn more examples in the next chapters.
Author: Aditya vardhan Vutturi