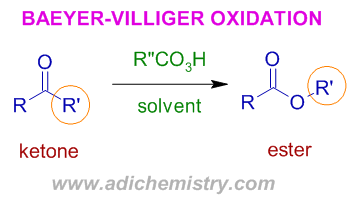
* The Baeyer villiger rearrangement involves oxidation of ketones to esters by using peroxy acids like MCPBA, TFPAA, H2O2.BF3 etc. Cyclic ketones furnish lactones (cyclic esters).

It is a regioselective rearrangement reaction involving preferential migration of alkyl/aryl group with greater migratory aptitude onto the electron deficient oxygen. In above case, the R' group is assumed to possess greater migratory aptitude and hence only one product is formed preferentially.
* The aldehydes may give carboxylic acids or formates. In the latter case, alcohols are finally formed due to hydrolysis of unstable formates under the reaction conditions.
* The Baeyer-villiger reaction involves the oxidative cleavage of C-C bond.
* The reagents which can be employed in Baeyer villiger oxidation include:
* Initially the peroxy group is added to the carbonyl carbon to give a Criegee like intermediate. Then one of the group attached to carbonyl carbon is migrated on to the electron deficient oxygen atom in a concerted step, which is the rate determining step.
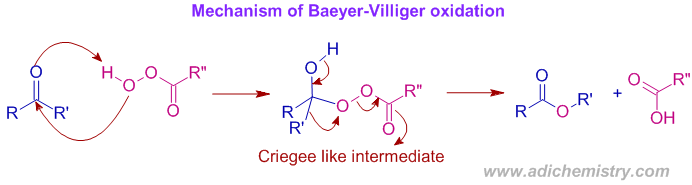
* In general, the reaction follows second order kinetics. The rate determining step may be addition step or most often the migration step. The Hammett ρ value is negative.
* The electron withdrawing groups (-I groups) on peroxy acids enhance the rate of the reaction.
* The Baeyer-villiger rearrangement reaction of unsymmetrical ketones is regioselective, with preferential formation of one regio-isomer. The substituents which can stabilize the positive charge can migrate readily. The migratory aptitude order in Baeyer villiger oxidation for various substituents is given below:
H > 3o-alkyl > cyclohexyl > 2o- alkyl > benzyl > aryl > 1o - alkyl > methyl.
Recall that the groups that release electrons easily either through positive inductive effect or delocalization can stabilize the positive charge.
* As the rearrangement is a concerted process, the configuration of the migrating chiral substituent is retained.
* In case of aldehydes, usually, the hydrogen atom is migrated preferentially and thus by furnishing carboxylic acids. But formates are also produced when the migrating group is other than the hydrogen. This is possible when the other substituent is a tertiary alkyl group or electron rich vinyl or aryl group (e.g. Dakin reaction).
* One of the competing reaction is the formation of epoxide when a double bond is present in the molecule, especially at low temperatures in neutral solvents.
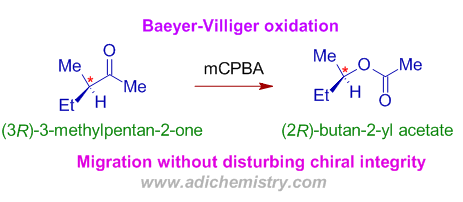
1) The Baeyer villiger oxidation of (3R)-3-methylpentan-2-one to (2R)-butan-2-yl acetate in presence of mCPBA, the preferential migration of more substituted secondary alkyl group is observed (regioselective) without disturbing its chiral integrity i.e., the configuration at the chiral carbon is retained in the product (stereospecificity).
2) Cyclic ketones furnish lactones. In the following illustration, cyclohexanone is converted to caprolactone (a starting compound for highly specialized polymers like polycaprolactone-a bio degradable polyester) in presence of CF3CO3H - TFPAA.
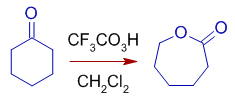
It is one of the best classic example showing the significance of Baeyer villiger reaction.
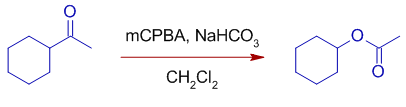
3) Cyclohexyl group migrates preferentially over methyl group as illustrated below. 1-cyclohexylethanone is rearranged to cyclohexyl acetate when treated with mCPBA + NaHCO3 in dichloromethane.
4) In the following example, the ketone, 1-(bicyclo[2.2.1]hept-2-yl)ethanone is rearranged to an ester, bicyclo[2.2.1]hept-2-yl acetate upon Baeyer villiger oxidation with mCPBA in dichloromethane and the subsequent hydrolysis of the ester gives the desired alcohol, bicyclo[2.2.1]heptan-2-ol . It is another example of regioselectivity and stereospecificity.
![baeyer villiger 1-(bicyclo[2.2.1]hept-2-yl)ethanone](baeyervilliger1-6.png)
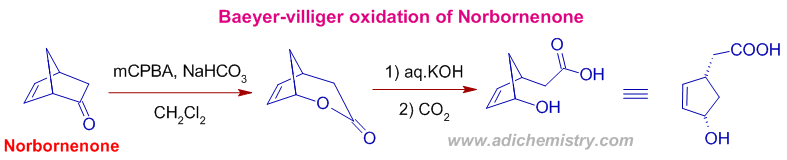
5) Baeyer villiger oxidation is preferred over the epoxidation of double bond by the peracid as illustrated in the following reaction. An oxygen atom is inserted into one of the rings of Norbornenone (bicyclo[2.2.1]hept-5-en-2-one) converting ketone into lactone. The double bond is intact.
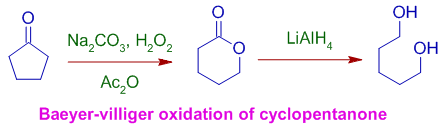
6) The lactone formed can be reduced to a dihydric alcohol. In the following example, the Baeyer villiger oxidation of cyclopentanone with hydrogen peroxide+sodiumcarbonate in acetic anhydride furnishes tetrahydro-2H-pyran-2-one, a lactone, which is further reduced to pentane-1,5-diol with LiAlH4.
7) The bacteria strain variants which produce BVMO can be employed in Baeyer villiger oxidation. In the following application, 3-phenylcyclobutanone is converted to 4-phenyldihydrofuran-2(3H)-one.

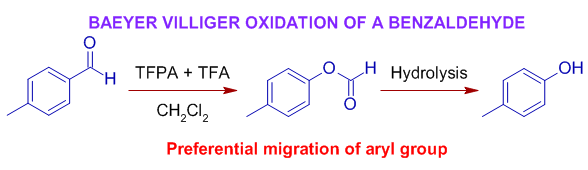
8) The Baeyer-villiger oxidation of following benzaldehde with TFPA+TFA in CH2Cl2 results in formation of a formate (4-methylphenyl formate) due to preferential migration of aryl group substituted by an electron donating group. But it undergoes hydrolysis under the reaction conditions to yield a phenol (4-methylphenol). This reaction is similar to that of Dakin reaction.
9) As illustrated below, the aldehyde group is oxidized to carboxylic acid due to preferential migration of the hydride ion. The aryl group with electronegative halogen groups has less migratory aptitude. Remember that the groups which can stabilize positive charge possess greater migratory aptitude.
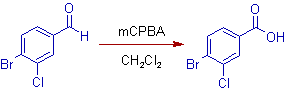
In above reaction, 4-bromo-3-chlorobenzaldehyde is converted to 4-bromo-3-chlorobenzoic acid.
10) The greater migratory aptitude of aryl group over the -CH2 group can be observed in the following example of Baeyer villiger oxidation of 2,3-dihydro-1H-inden-1-one to 3,4-dihydro-2H-chromen-2-one by using sodium percarbonate in trifluoroacetic acid.
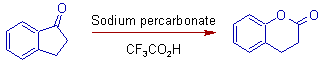
11) The -CH2 group is migrated preferentially in the following reaction. The -CH-CF3 group has less migratory aptitude due to electron withdrawing nature. It is illustrated in the following oxidative rearrangement of 2-(trifluoromethyl)cyclohexanone to 3-(trifluoromethyl)oxepan-2-one by using a mixture of TFPAA+TFAA in dichloromethane.
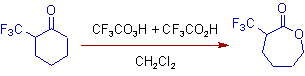
1) The incorrect statement regarding Baeyer villiger oxidation is: (AdiChemistry Online CSIR NET practice test 2019)
(A) Ketones are converted to esters.
(B) The configuration of migrating group is retained.
(C) It involves concerted rearrangement groups attached to carbonyl carbon onto electron rich oxygen.
(D) There is oxidative cleavage of C-C bond.
Answer: C
2) The major product expected when (2S,3S)-2,3-dimethylcyclohexanone is treated with mCPBA is:
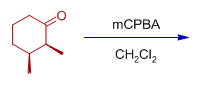

Answer: B
Explanation: Secondary alkyl group has greater migratory aptitude than the primary. There is retention of configuration of migrating group.
3) The major product formed when p-menthone [(2R,5S)-5-methyl-2-(propan-2-yl)cyclohexanone)] is treated with H2SO5 in presence of Na2HPO4 is :
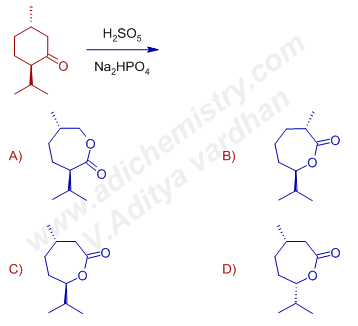
Answer: C
Explanation: Again the secondary ring carbon is migrated preferentially over the primary ring carbon with retention of configuration in the migrating group which clearly shows the concerted nature of rearrangement step.
4) The major product formed in the Baeyer villiger oxidation of following molecule with steroidal skeleton is:
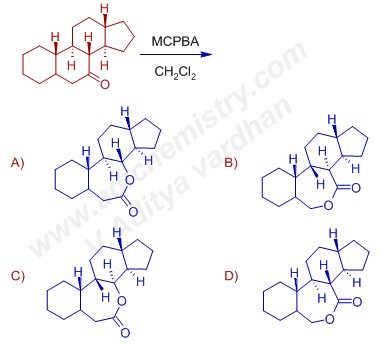
Answer: A
5) The major product formed when 2,2-dimethyl-1-phenylpropan-1-one is oxidized with K2SO5 on hydrated silica is:
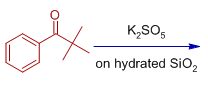
(A) phenyl 2,2-dimethylpropanoate
(B) tert-butyl benzoate
(C) Isopropyl benzoate
(D) ortho tert-butyl benzoic acid
Answer: B
Explanation: Structure of the major product is:
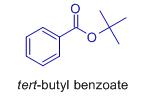
The tertiary alkyl group is migrated preferentially over the phenyl group.
6) The correct statement about Baeyer villiger oxidation reaction among the following is:
(A) The migratory aptitude in Baeyer-Villiger oxidation is in the order H > tert-alkyl > sec-alkyl > phenyl > primary alkyl > methyl
(B) The electron withdrawing groups (-I groups) on peroxy acids enhance the rate of the reaction.
(C) Baeyer villiger oxidation of unsymmetrical ketones is regioselective.
(D) All are correct statements.
Answer: D
7) The major product formed when (1R,5S)-bicyclo[3.2.0]hept-3-en-6-one is treated with Baeyer villiger monoxygenase is:
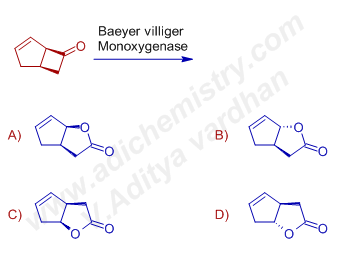
Answer: A
8) The major product formed in the Baeyer villiger oxidation of camphor with mCPBA and NaHCO3 is:
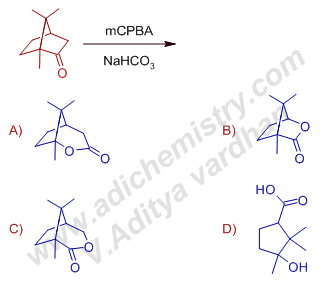
Answer: A
Explanation: Baeyer Villiger oxidation of camphor with mCPBA in buffer conditions, gives the major product (65%) that is formed due to migration of a tertiary bridgehead carbon rather than a methylene group. The Criegee intermediate can be formed either by exo or endo attack of peroxy acid. If it attacks in exo fashion, the migration of bridge head carbon is more favored since it goes through chair shaped transition state.
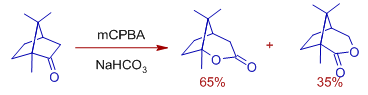
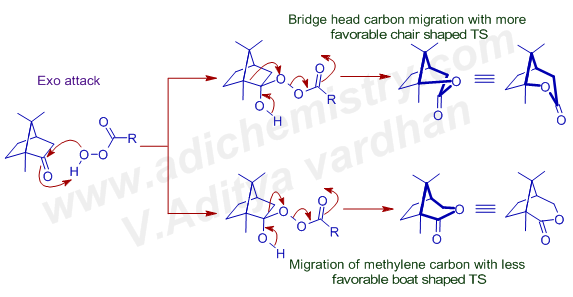
9) phenyl(4,7,7-trimethylbicyclo[2.2.1]hept-1-yl)methanone is treated with Trifluoroperoxyacetic acid, CF3CO3H to give 'X'. What is 'X'?
![phenyl(4,7,7-trimethylbicyclo[2.2.1]hept-1-yl)methanone](bv-2-9q.png)
Answer: D
Common Data for Questions 10 and 11 :
(GATE 2009)
DATA: An organic compound
'X' (C9H10O) exhibited the following spectral data :
IR : 1680 cm–1
1H NMR : δ 7.8(2H, d, J = 7.5 Hz), 7.2(2H, d, J =
7.5 Hz) and 2.7(3H, s), 2.4(3H, s)
The compound 'X' on treatment with m-chloroperbenzoic acid produced two isomeric
compounds Y(major) and Z (minor).
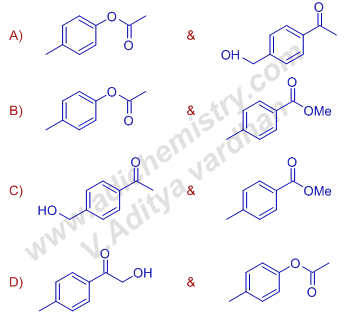
10) Compounds Y and Z, respectively, are
Answer: B
Explanation: 'X' with the formula (C9H10O) with DBE = 5 suggests the presence of a benzene ring.
The peaks in 1H NMR spectrum at δ 7.8(2H, d, J = 7.5 Hz), 7.2(2H, d, J = 7.5 Hz) not only confirms this but also indicate para substitution. The singlets at 2.7(3H, s) & 2.4(3H, s) show the presence of two methyl groups.
The ninth carbon must be a carbonyl carbon. The strong peak at 1680 cm–1 in IR spectrum supports this.
Treatment of 'X' with metachloroperbenzoic acid, mCPBA results in Baeyer Villiger oxidation. Since the ketone is unsymmetrical, two esters are possible. However, as the migratory aptitude of phenyl ring is greater than that of methyl group, the major product is ester of acetic acid.
Note: Double Bond Equivalent (DBE) = C+1-(H/2)-(X/2)+(N/2), where X = halogen
11) Compounds Y and Z can be differentiated by carrying out basic hydrolysis, because
A) Y produces 4-methyphenol and Z is unaffected
B) Y preoduces 4-methylphenol and Z produces 4-methylbenzoic acid
C) Y is unaffected and Z produces 4-methylbenzoic acid
D) Y is unaffected and Z produced 4-methylphenol
Answer: B
12) Baeyer villiger rearrangement of adamantanone is carried out in presence of:
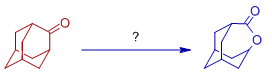
A) CF3CO3H, Na2CO3.1.5H2O2
B) Zymase, O2
C) POCl3
D) O3, hν
Answer: A
13) Which of the following reactions would result in ring expansion?
A) Favoroskii reaction
B) Beckmann rearrangement
C) Baeyer villiger oxidation
D) All of the above
Answer: B & C
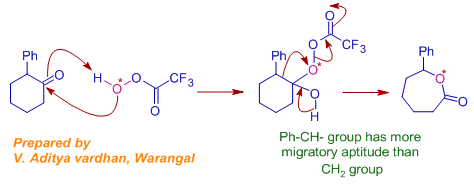
14) 2-phenylcyclohexanone is treated with isotopically labelled peracid as shown below. Choose the correct product formed in this reaction is:
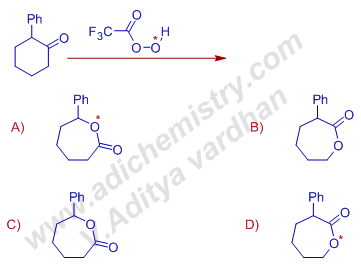
Answer: A
Explanation: It is Baeyer-villiger oxidation. The isotopically labelled oxygen will be the electron deficient oxygen and hence is retained in the product.
15) Acetophenone can be converted to phenol by reaction with:
(A) conc. HNO3
(B) I2 and NaOH
(C) mCPBA followed by base catalyzed hydrolysis
(D) mCPBA followed by heating with AlCl3
Answer: C
16) Acetophenone can be converted to phenol by reaction with:
(A) conc. HNO3
(B) I2 and NaOH
(C) mCPBA followed by base catalyzed hydrolysis
(D) mCPBA followed by heating with AlCl3
Answer: C
1) Draw the major and minor product that are formed when bicyclo[3.2.1]oct-6-en-2-one is subjected to conditions for the baeyer–villiger reaction.
2) What is mCPBA? What are its applications?