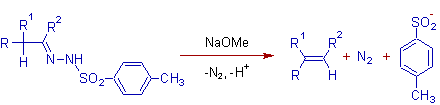
* In the Bamford-Stevens reaction, the tosyl hydrazones (p-Toluenesulfonyl hydrazones) of aliphatic aldehydes or ketones furnish more substituted alkenes when treated with strong bases like NaOMe, NaH, LiH, NaNH2 etc.
* The reaction may be performed either in protic solvents like glycols or in aprotic solvents like ethylene glycol dimethyl ether.
* Both the Bamford-Stevens reaction and the Shapiro reaction afford alkenes from tosyl hydrazones.
* In case of Bamford-Stevens reaction, the more substituted alkene is formed as the thermodynamic product.
* Whereas in Shapiro reaction, the less substituted alkene is formed as the kinetic product. This reaction employs bases such as alkyllithiums and Grignard reagents.
MECHANISM OF BAMFORD-STEVENS REACTION
* The mechanism involves two steps. Initially, the reaction of tosyl hydrazone with a strong base leads to a diazo compound, which can be isolated in some cases.
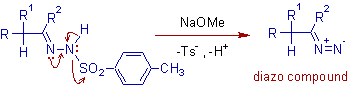
* The diazo compound may follow either one of the two pathways depending on the reaction conditions. In protic solvents, the reaction proceeds via formation of carbenium ion, whereas in aprotic solvents, the reaction proceeds via a carbene.
In protic solvents:
* In protic solvents, the diazo compound abstracts a proton from the solvent and thus by forming a diazonium ion, which subsequently loses dinitrogen to give a carbenium ion. Finally, a mixture of E & Z alkenes is formed from the carbenium ion through loss of a proton.
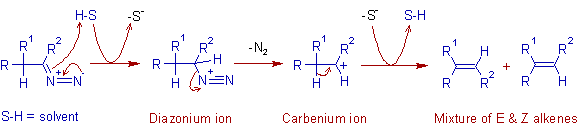
* However, carbenium ions can easily undergo a Wagner–Meerwein rearrangement, and hence the corresponding rearranged alkenes may be formed as side products in protic solvents.
Note: A carbenium ion is a trivalent carbocation. Whereas, the carbocation with five coordinated carbon is nowadays referred to as a carbonium ion.
In aprotic solvents:
* In aprotic solvents, the diazo compound loses dinitrogen and gives a carbene, which undergoes a faster 1,2-hydrogen shift to furnish a Z-alkene predominantly.
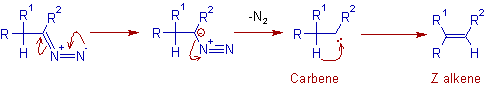
* The desired alkene is obtained in high yield in aprotic solvents.
ILLUSTRATIONS
1) Bamford-Stevens reaction of tosyl hydrazone of 2-methylcyclohexanone affords more substituted 1-methylcyclohexane.

Whereas, the Shapiro reaction conditions lead to less substituted 3-methylcyclohexane.
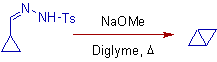
2) The Bamford-Stevens reaction of tosyl hydrazone of cyclopropane carbaldehyde furnishes bicyclobutane: a special case.