* The Beckmann rearrangement is an acid catalyzed rearrangement of an oxime to an N-substituted amide.
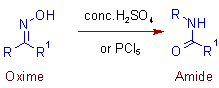
* Conc.H2SO4, HCl, PCl5, PCl3, SOCl2, ZnO, SiO2, PPA (Poly phosphoric acid) etc., are commonly employed in Beckmann rearrangement.
* Mostly applied for ketoximes.
* Aldoximes are less reactive.
* Cyclic oximes give lactams (cyclic amides).
FIND STUDY MATERIAL AND GET ACCESS TO ONLINE MODULES FOR CSIR NET & GATE EXAMS
MECHANISM OF BECKMANN REARRANGEMENT
* Initially the -OH group of the oxime is protonated. Then 1,2 shift of alkyl group (R1) onto electron deficient nitrogen and the cleavage of N-O bond occurs simultaneously.
Always the alkyl group which is 'anti' to the -OH group on nitrogen undergoes 1,2 shift which indicates the concerted nature of the beckmann rearrangement.
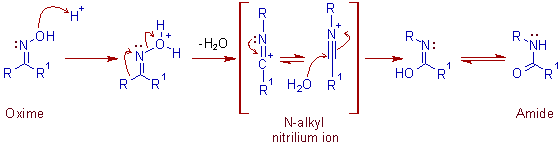
Comment: The migration of hydrogen is seldom observed. Hence the N-unsubstituted amides cannot be obtained by beckmann rearrangement reaction.
ILLUSTRATIONS
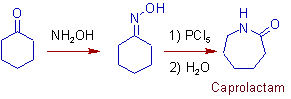
1) Industrial conversion of cyclohexanone to caprolactam, which is used in the manufacture of Nylon-6, involves Beckmann rearrangement.
2) The relative migratory aptitudes of different groups in Beckmann rearrangement is illustrated below.
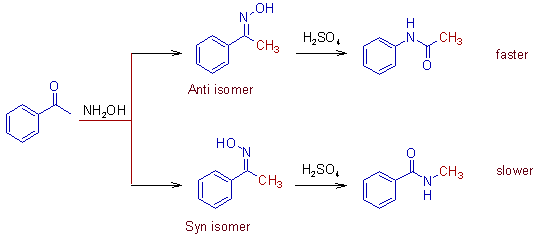
The 1,2 shift of phenyl group is faster than that of alkyl groups. It is due to formation of phenonium ion. Hence the anti isomer reacts faster than the syn isomer.

Note: Learn how to assign syn and anti descriptors to oximes at Geometrical isomerism page.
BECKMANN FRAGMENTATION
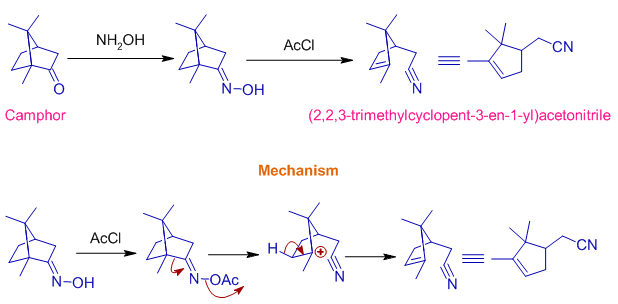
Beckmann fragmentation occurs when the migrating group departs from the intermediate and thus by furnishing a nitrile. It occurs when the migrating groups can stabilize the positive charge very well (like tertiary, benzyl etc.)
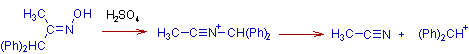
The carbocation formed after breaking the bond is a very stable one.
Another example is Beckmann fragmentation of Camphor oxime when treated with dilute H2SO4 or acetyl chloride (AcCl) to furnish an unsaturated nitrile, (2,2,3-trimethylcyclopent-3-en-1-yl)acetonitrile as shown below.