* In Birch reduction, aromatic rings are reduced to 1,4-dienes by alkali metals in liquid ammonia.
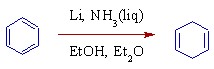
* Commercial ammonia often contains iron as impurity. Therefore, it is often necessary to distill the ammonia before using it in the Birch reduction.
* The reaction is carried out at -33oC (boiling point of ammonia). Co-solvents like Et2O, THF, DME etc., are added to improve the solubility of organic compounds at this temperature.
MECHANISM OF BIRCH REDUCTION
Comments:
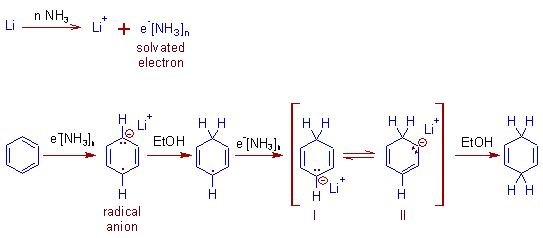
* The alkali metals dissolve in liquid ammonia by forming solvated electrons which are responsible for the reduction.
* The second protonation is almost always occurs at a site para to the first protonation site.
* Hence in the final step of protonation, thermodynamically less stable but kinetically favored 1,4-diene is formed predominantly (about 80%). However thermodynamically more stable 1,3-diene(conjugated) is also formed in minor quantities and may become major product under certain conditions.
* The formation of 1,4-diene in Birch reduction can also be explained by "the principle of least motion", which states that the reaction that involves the least change in atomic positions or electronic configuration is favored
* Alcohols (EtOH or t-BuOH) are used as protonating agents. They also suppress the formation of amide, NH2- ion, which may otherwise isomerize the 1,4-diene to more stable 1,3-diene.
* Under the reaction conditions (below 240 K), the alcohols do not react with the metals.
* Relatively the Birch reductions using Li metal are very faster.
REGIOSELECTIVITY IN BIRCH REDUCTION
The positions of protonation on substituted benzenes depend on the nature of the group as illustrated below.
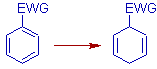
* EWG: The electron-withdrawing groups promote ipso & para reduction. These groups activate the ring towards birch reduction. Initially the protonation occurs para to the EWG.
E.g. -COOH, -CONH2, aryl group etc.,
* EDG: The electron-donating groups promote ortho & meta reduction. They deactivate the ring for overall reduction compared to the EWG.
E.g. -R, -OR, -NR2, -SR, PR2, -CH2OH, -CHO, -C(O)R, CO2R etc.,
The -CHO, -C(O)R, CO2R act as electron donating groups because they are reduced to -CH2O- prior to the reduction of the ring.
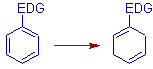
ILLUSTRATIONS
1) Naphthalene can be reduced to 1,4,5,8-tetrahydronaphthalene by using Birch reduction conditions.
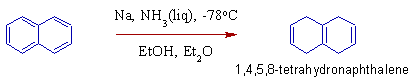
2) In the birch reduction of benzoic acid, the protonation occurs at ipso and para positions relative to -COOH group on the benzene ring.
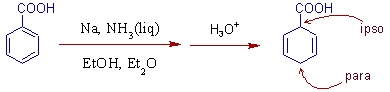
comments:
* The reason is electron withdrawing groups stabilize the radical anion at ipso and para positions.
* The carboxylate ion, -COO- formed during the reaction is electron rich and hence cannot be reduced.
* The reduction is also possible even without the presence of alcohol due to strong activation by -COOH group.
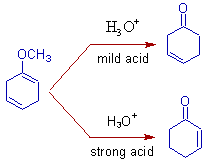
3) Protonation occurs at ortho and meta positions of benzene ring incase of anisole and thus by giving more substituted double bond. It is due to the fact that, electron donating groups stabilize the radical anion at ortho and meta positions.
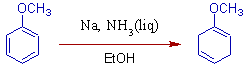
* The above product can be hydrolyzed to β,γ-unsaturated ketone in presence of mild acid. However, α,β-unsaturated ketone is formed due to isomerization in vigorous acid catalytic conditions.
4) The electron donating groups deactivate the ring towards birch reduction. This is exemplified below.
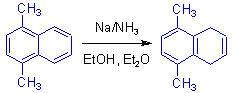
The reduction occurs in the unsubstituted ring of naphthalene.
5) But with aniline derivatives (even though electron donating), the conjugated enamines are formed directly as the major products (No need of acid catalyst).
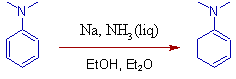
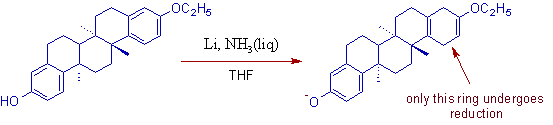
6) In case of phenols, the birch reduction is not possible. It is because, phenolic function becomes phenolate ion under the reaction conditions (basic) and does not react further.
7) The carboxyl groups have a dominating directive influence than other groups.
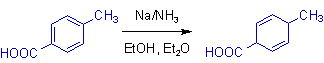
8) The additive effect of directive influences of alkoxy & alkyl groups can be seen in the following reaction.
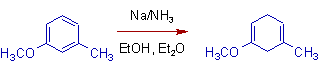
9) The carbanion formed during birch reduction can undergo alkylation.
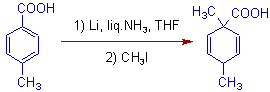
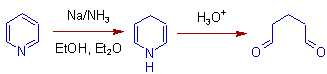
10) Electron deficient heterocyclic aromatic compounds can also be reduced in Birch reduction.
E.g. Pyridine gives 1,4-dihydropyridine, which can be further hydrolyzed to 1,5-dicarbonyl compound.
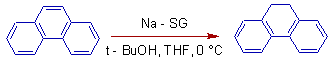
11) Nowadays alkali metals encapsulated in nano structured oxides like silica gel are used instead of liquid ammonia-metal solutions. E.g. sodium in silica gel (Na-SG). In the following example, phenanthrene is reduced to 9,10-dihydrophenanthrene