* The Finkelstein reaction involves the exchange of one halogen for another, especially, in primary alkyl halides. It is used to synthesize one alkyl halide from another.
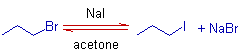
In the classical version of Finkelstein reaction, a primary alkyl halide, RX is treated with an alkali metal halide, like NaX' or KX', in excess in acetone. The halogen, X in alkyl halide is replaced by X' through an SN2 mechanism.
| Acetone | ||
| R-X + NaX' | <=========> | RX' + 2NaX |
Where R = alkyl group; X & X' are different halogens (usually X = Cl or Br, X' = F or I).
* The halide exchange is a reversible reaction. The reaction is driven to completion by taking the advantage of differential solubility of metal halide salts in acetone solvent.
The solubility of sodium chloride or sodium bromide in acetone is much less than the solubility of sodium iodide. During the reaction of an alkyl chloride or bromide with sodium iodide in acetone, the formed sodium chloride or bromide precipitates out from the solution and is thus removed from equilibrium and drives the reaction to completion.. Hence Finkelstein reaction is usually employed to prepare alkyl iodides which are otherwise difficult to prepare directly.
* The success of this reaction not only depends on the solubility of the metal halide but also depends on:
i) the nucleophilicity of the attacking halide ion,
ii) the nature of the leaving group,
iii) the stability of carbon-halogen bond in the newly formed alkyl halide and
iv) the reactivity of alky halide.
* The F- is a poor leaving group and forms stable C-F bond. Therefore it is possible to exchange other halogen groups with fluoride by using KF or AgF or gaseous HF in presence of crown ethers, which is used to improve the solubility of the metal fluoride. The quaternary ammonium fluorides in aprotic solvents can also be used. Thus Finkelstein reaction is also used to prepare alkyl fluoride.
* The alkyl bromides are more reactive than corresponding chlorides.
* The allyl, benzyl and α-carbonyl halides are more reactive.
* The secondary, tertiary, vinyl and aryl halides are less reactive in Finkelstein reaction. But the reactivity of secondary and tertiary halides can be improved by using catalysts like ZnCl2, FeCl3 etc., in solvents like CS2.
* The electron donors on the alkyl halide increase the rate of the reaction, whereas the electron withdrawing groups tend to decrease the rate.
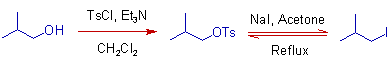
* In the modified version of Finkelstein reaction, an alcohol is first converted to a tosylate or mesylate and then treated with a metal halide to get the desired alkyl halide. This reaction works since the tosylate and mesylate are an excellent leaving groups.
ILLUSTRATIONS
1) In the following Finkelstein reaction, the propyl bromide is converted to propyl iodide.
2) In the following Finkelstein reaction, TetraButyl Ammonium Fluoride (TBAF) is used to prepare (fluoromethyl)cyclohexane.
![]()
3) The halogen exchange for a tertiary halide can be achieved by using ZnCl2 as catalyst in CS2.
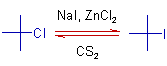
4) An alcohol can be converted to a tosylate and then to an alkyl iodide as illustrated in the following scheme of reactions. The tosylate is formed by reacting the alcohol with Tosyl chloride in presence of Et3N in dichloromethane. This is a modified version of Finkelstein reaction.