* In Williamson's synthesis, an ether is prepared by the nucleophilic substitution (typically SN2) of organic halide with an alkoxide ion.
| R-O- | + | R'-X | -----------------> | R-O-R' | + X- |
| Alkoxide
ion |
Halide | Ether |
* The alkoxide ion is generated in situ by treating an alcohol with a metal or a strong base.
* Williamson's synthesis follows bimolecular nucleophilic substitution (SN2) pathway.
* Both symmetrical or unsymmetrical ethers can be prepared.
* In Williamson's synthesis, the nature of alkoxide ion is less important. It may be primary or secondary or tertiary.
* But due to strongly alkaline conditions, dehydrohalogenation (elimination) is a side reaction. Hence the yields are relatively better with methyl or primary alkyl halides only.
The yields are affected when halides contain β-hydrogen. Elimination products are formed exclusively with tert-halides.
MECHANISM OF WILLIAMSON'S SYNTHESIS
* It is a typical SN2 reaction. The alkoxide ion attacks the carbon atom containing the halogen atom from the back side. The bond making and breaking occurs simultaneously in the transition state.
![]()
ILLUSTRATIONS
1) A classical example of Williamson's synthesis can be seen in the preparation of diethyl ether as shown below. Note that, initially, the sodium ethoxide is generated by treating ethyl alcohol with sodium metal.
| Na | C2H5Cl | |||
| C2H5OH | --------------> | C2H5O-Na+ | ------------------> | C2H5OC2H5 |
| -NaCl |
2) A cyclic ether is formed in the following reaction.
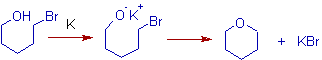
3) In the following Williamson's synthesis, propene is also formed in good quantities due to elimination side reaction.
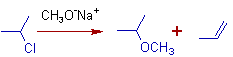
4) An epoxide can be synthesized from a halohydrin using Williamson's reaction.
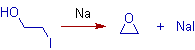
5) Phenoxide ions can be employed to get aromatic ethers.
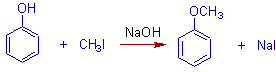
Note: Halobenzenes do undergo nucleophilic substitution and hence they cannot be not used in Williamson's synthesis.