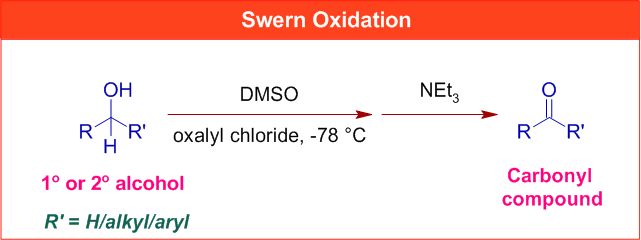
In Swern oxidation, the primary or secondary alcohols are oxidized to aldehydes or ketones respectively by treating them with dimethyl sulfoxide (DMSO) activated by oxalyl chloride at low temperatures (-78 oC to -60 oC) and then with an organic base like triethyl amine.
* Further oxidation of aldehydes to carboxylic acids is not possible under Swern oxidation conditions.
* The Swern oxidation is the best alternative to the use of carcinogenic chromium based oxidizing agents.
* The reaction must be performed below -60 oC to avoid the formation of side products like mixed thioacetals. However the use of trifluoroacetic anhydride instead of oxalyl chloride, (COCl)2 allows the reaction to be warmed up to -30 oC.
* The dimethyl sulfide (DMS) is formed as a byproduct. It has very unpleasant smell. Hence the glassware is usually rinsed with sodium hypochlorite to oxidize it.
To avoid not only the formation of volatile and malodorous dimethyl sulfide but also to facilitate the workup, the alternative sulfoxides like dodecyl methyl sulfoxide or sulfoxides bound to polymers can be used.
MECHANISM OF SWERN OXIDATION
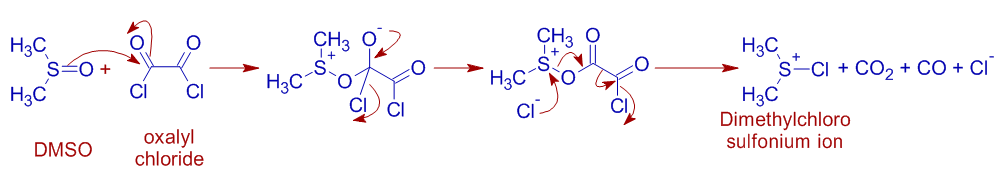
* The DMSO reacts with oxalyl chloride and gets converted to a reactive species, dimethylchlorosulfonium ion.
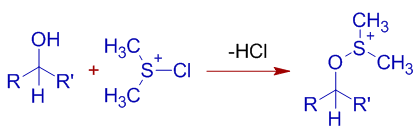
* The dimethylchlorosulfonium ion, thus formed, reacts with the alcohol to give an alkoxysulfonium ion.
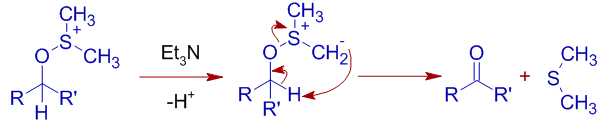
* The deprotonation of the alkoxysulfonium ion using a base furnishes sulfur ylide, which undergoes β-elimination through a five membered cyclic transition state to give a carbonyl compound and dimethyl sulfide.
ILLUSTRATIONS
1) The hexan-1-ol is conveniently oxidized to hexanal in Swern oxidation.
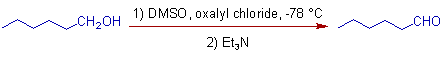
2) The oxidation of alcohols occurs selectively in presence of disulfide in Swern conditions. The sulfurs are not oxidized.
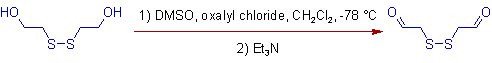
3) In some cases, the side reactions like α-epimerization and migration of double bonds into conjugation with newly formed carbonyl group are possible with the use of triethylamine as the base. To mitigate these reactions, a bulkier base like diisopropylethylamine (iPr2NEt) also known as, Hünig's base can be employed.
E.g.
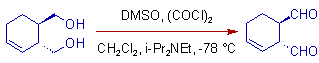
However the double bond can be migrated when triethylamine is used.
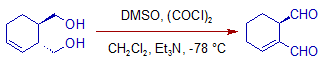
4) Some times the use of N-ethylpiperidine as a base may improve the yields in Swern oxidation.
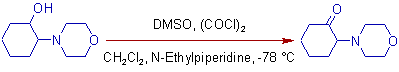
SWERN OXIDATION PRACTICE QUESTIONS MCQ CSIR NET GATE SET IIT JAM CHEMISTRY
1) Which of the following reagents or reagent sequences would convert cyclopentanol into 2- oxocyclohexanone (a lactone)?
1) i) oxalyl chloride in DMSO at -60 oC; ii) Et3N; iii) LiAlH4
2) Ag+ in aqueous NH3
3) i) oxalyl chloride in DMSO at -60 oC; ii) Et3N; iii) CF3CO3H
4) i) HIO4; ii) O3 ; iii) Me2S
Answer: 3
Explanation: The -OH group in cyclopentanol is oxidized to a ketone, cyclopentanone under Swern oxidation conditions. It is then subjected to Baeyer villiger oxidation using CF3CO3H to get a lactone (a cyclic ester).