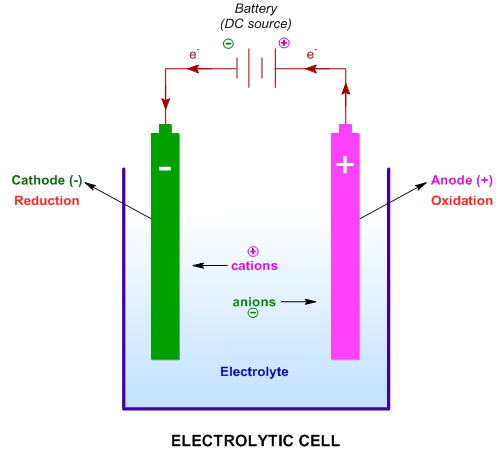
Electrolysis definition: It involves dissociation (lysis) of an electrolyte by using a direct electric current. In this process, an electromotive force is employed to carry out a non-spontaneous redox chemical reaction in an electrolytic cell using proper electrodes.
On this page, you will learn what is electrolysis used for in chemistry, what are electrodes, electrolytic cell construction, and about electrolysis reactions.
An electrolytic cell is used to perform electrolysis. It is provided with two electrodes, that are connected to different ends of the DC electric source. The electrode connected to the positive end is referred to as anode and that which is connected to the negative end is referred to as the cathode.
Electrodes help in the conduction of electrons into and out of the cell as well as provide the surface for electrode reactions.
What are electrodes made of?
They are made up of electric conductors like metals or graphite. They may be in the form of rods or as a surface coating on the rods of other material or as a coating on the inside surface of the electrolytic cell.
In general platinum and graphite are used in making electrodes. They are inert and do not participate in the electrolysis reactions. However, the former is very costly while the latter is cheaper.
In the electrolytic cell, the anode is denoted by a positive sign while the cathode is denoted by a negative sign.
These signs are reversed in case of Galvanic cells.
However, one should keep in mind that irrespective of whether it is an electrolytic cell or a galvanic cell, at the anode, an oxidation half reaction takes place while at the cathode the other half-reaction - reduction occurs.
The electrodes may be divided into two types depending on how they are involved in the electrolytic process.
i.e.
i) Inert electrodes - which do not enter into the electrolytic chemical reactions. The electrodes made up of Noble metals like platinum are used as inert electrodes.
ii) Active electrodes - which take part in the reactions of electrolysis. These are either dissolved into the electrolyte or a substance is deposited on them.
The cell is filled with the melt or the aqueous solution of the electrolyte and is subjected to potential difference sufficient to drive the electrolysis. The electrolyte is the substance that furnishes free ions either in the molten state or in the aqueous medium.

What is electrolysis in chemistry?
During electrolysis, the free ions furnished by the electrolyte are migrated towards oppositely charged electrodes and are discharged under electric potential.
i.e.
* Cations migrate towards the cathode and are reduced.
* Anions migrate towards the anode and are oxidized.
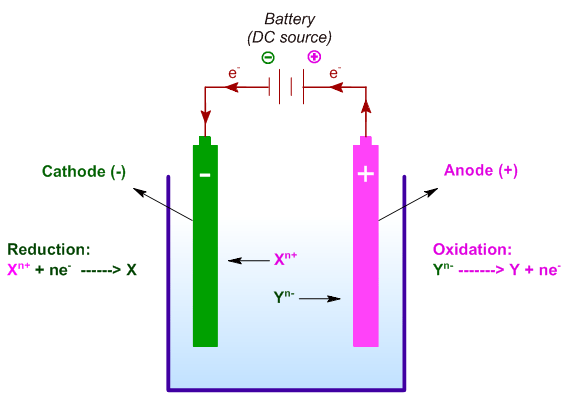
E.g. Let us take a hypothetical electrolyte, XY, which is ionized as follows:
Ionization:
XY -------> Xn+ + Yn-
The cation, Xn+ migrates towards the cathode and gets discharged to 'X' by reduction i.e. by gaining electrons. Whereas, the anion, Yn- moves towards the anode and is oxidized to 'Y' by losing electrons.
At cathode:
Xn+ + ne- -----------> X (reduction half reaction)
At anode:
Yn- -----------> Y + ne- (oxidation half reaction)
The complete electrolytic reaction can be written as:
XY -------> X + Y
Thus electrolysis is a redox reaction. The reduction half-reaction occurs at the cathode, whereas the oxidation half-reaction occurs at the anode. The entire process, otherwise non-spontaneous, is driven by the electromotive force.
However, sometimes the solvent molecules or the electrodes may also participate in the electrolysis instead of the ions furnished by the electrolyte.
The electrolytes are ionic compounds, which furnish free ions in the molten state. When ample electromotive force is applied, these free ions start migrating towards the electrodes and get discharged.
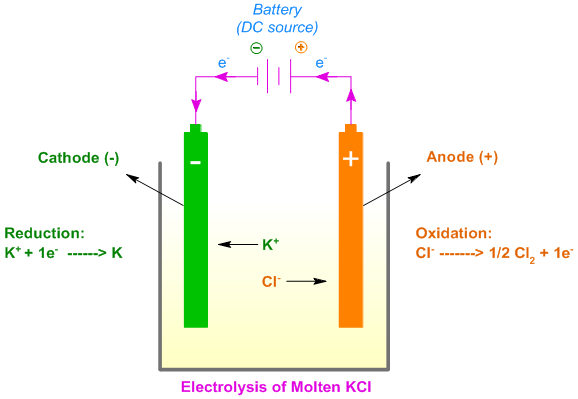
For example, when molten KCl is subjected to electrolysis using inert electrodes, the K+ ions migrate towards cathode and get reduced to potassium metal, K by gaining electrons. The Cl- ions migrate towards the anode and get oxidized by losing electrons to liberate Cl2 gas.
Ionization of electrolyte:
KCl -------------------> K+ + Cl-
At cathode:
K+ + 1e- --------------> K (reduction half reaction)
At anode:
Cl- ---------------------> ½Cl2 (oxidation half reaction)
Complete electrolytic reaction:
KCl -------------------> K + ½Cl2
When the electrolyte is dissolved in water, the ions furnished will be in the hydrated condition; and hence may or may not be discharged at the electrodes depending on the nature of ions, the concentration of a solution, nature of electrodes, etc.
In general, the ions of active metals like alkali and alkaline earth metals do not undergo reduction in the presence of water. Instead, water molecules undergo reduction to liberate dihydrogen gas, H2.
For example, when a moderately concentrated aqueous solution of KCl is subjected to electrolysis using inert electrodes, only Cl- ions get discharged at the anode. Whereas, the K+ ions cannot be reduced in the presence of water. Instead, water molecules undergo reduction to give dihydrogen (H2) gas.
However, the ions of less reactive transition metal series can be discharged in the aqueous medium.
For example, the electrolysis of aqueous CuCl2, copper is deposited at the cathode along with the liberation of chlorine gas at the anode.
Some stable oxo anions like sulfate, phosphate, carbonate, nitrate, etc. also do not participate in the electrolytic reactions. Alternatively, the water molecules undergo oxidation at anode and thus by liberating dioxygen gas, O2.
When an aqueous solution of AgNO3 is electrolyzed, the silver ion, Ag+ is discharged at the cathode. But the nitrate ion, NO3- cannot be oxidized at the anode. In place of it, the water molecule is oxidized to liberate dioxygen gas, O2 at anode.
Regardless of the nature of the ions, only water molecules undergo electrolysis when dilute solutions are used.
For example, when a very dilute aqueous solution of NaCl is subjected to electrolysis, dihydrogen and dioxygen gases are liberated at cathode and anode respectively. In this case, NaCl helps in improving the electrical conductivity of water, which was otherwise a poor conductor.
The electrodes become active when the metal used in one or either of them and the cation in the electrolyte belong to the same element. The electrodes now enter into the electrolytic reaction. At anode, the metal undergoes oxidation and dissolves in the electrolyte, while the metal is deposited on cathode due to reduction of metal ions.
For example, when an aqueous solution of silver nitrate, AgNO3 is electrolyzed using silver electrodes, some amount of silver in the anode is dissolved into the solution and the same amount of silver is deposited on cathode.
This fact can be used in the purification process of impure samples of metals.
1) In metallurgy, electrolysis process is used to extract metals from their ores and also in the refining process of metals. The subject that deals with electrolytic processes in metallurgy is generally referred to as electrometallurgy.
Electrolysis examples: Metals like sodium, potassium, aluminium, zinc etc. are obtained from electrolysis of electrolytes containing these metals. For strongly electropositive metals, molten electrolyte is subjected to electrolysis and for other less electropositive metals, aqueous solutions can be electrolysed.
The metals can be refined by electrolysis process generally referred to as electrorefining. In general, the metals are deposited at cathode. An impure sample of metal solution is electrolysed using cathode made up of same metal in pure state so that the metal from solution is deposited on it during electrolysis.
2) Not only metals, but also non metals like chlorine, fluorine, oxygen, hydrogen, etc. can be obtained by electrolysis process.
3) Many important chemicals like caustic soda (sodium hydroxide), potassium chlorate, etc. can be prepared by electrolysis process on industrial scale.
4) Electrolysis can be used in electroplating, electrotyping, and electroforming. The electroplating is used to improve corrosion resistance and in the making of ornaments.
5) Determination of equivalent weights of elements.
6) Determination of thickness of coated layer on a plate.
7) In the hair removal process.
Question-1) When water is electrolyzed, the gas liberated at cathode, is
a) Dioxygen
b) Dihydrogen
c) Carbon dioxide
d) No gas is liberated
Answer: b
Explanation: The H+ ions (cations) migrate towards cathode and get discharged by gaining electrons (reduction) and thus dihydrogen gas is liberated.
H+ + e- -----> 1/2 H2
Question-2) What happens to electrodes in electrolysis?
a) Active electrodes especially those acting as anodes may dissolve in the electrolyte.
b) Not much will happen to inert electrodes like platinum or graphite except scaling over them and deformation with time.
c) Active cathode may gain weight during electrolysis
d) All of the above
Answer: d
Explanation: See Types of electrodes
Question-3) What compounds are used in electrolysis?
a) Insulators
b) Semi conductors only
c) Electrolytes
d) All of the above
Answer: c
Question-4) Why can graphite be used as an electrode during electrolysis ?
a) It is an insulator
b) It is a semiconductor
c) It is good electric conductor
d) It dissolved in water
Answer: c
Question-5) What is the electrolyte used in an Edison cell?
A) NaCl
B) KOH
C) sulfuric acid
D) CaCl2
Answer: B
Question-6) When the sample of copper with zinc impurity is to be purified by electrolysis, the appropriate electrodes are [IIT MAIN (AIEEE) 2002]
| Cathode | Anode | |
| A) | Pure zinc | Pure copper |
| B) | Impure sample | Pure copper |
| C) | Impure zinc | Impure sample |
| D) | Pure copper | Impure sample |
Answer: D
Explanation: Copper will be deposited by reduction at cathode from the impure sample. Cathode is madeup of a thin sheet of pure copper.
Question-8) Which of the following will not conduct electricity in aqueous solution [AMU 1982 & 1983]
A) Copper sulphate
B) Sugar
C) Common salt
D) None of these
Answer: B
Explanation: Sugar (sucrose) is a non-electrolyte and cannot furnish ions for electrical conduction in water.
Question-9) Electrolytes when dissolved in water dissociates into ions because [CPMT 1974]
A) They are unstable
B) The force of repulsion increases
C) The water dissolves it
D) The forces of electrostatic attraction are broken down by water
Answer: D
Question-10) Which one of the following metals could not be obtained on electrolysis of aqueous solution of its salts [IIT 1990]
A) Cr
B) Mg
C) Cu
D) Ag
Answer: B
Explanation: Mg is a highly electropositive metal and hence can reduce water during electrolysis.
Question-11) On the electrolysis of aqueous solution of sodium sulphate, on cathode we get [MP PMT 1992]
A) Na
B) SO3
C) SO2
D) H2
Answer: D
Explanation: Na+ ion cannot be reduced at cathode in aqueous solutions since sodium is a highly electropositive metal. Hence instead of it dihydrogen gas is liberated at cathode as a result of reduction of H+ ions. At anode, dioxygen gas is liberated. The sulphate ions are very stable and therefore cannot be oxidized at anode.
Question-12) The current flow through electrolyte is due to the movement of
(A) Ions
(B) Holes
(C) Electrons
(D) None of the above
Answer: C
Question-13) Which of the following compound(s) do not conduct electricity in their molten state?
(A) MgCl2
(B) MgF2
(C) BeCl2
(D) BeF2
Answer: C & D
Explanation: These are covalent compounds and do not provide ions for flow of electricity.
Question-14) In the electrolytic cell, flow of electrons is from [IIT Main 2003]
A) Cathode to anode in solution
B) Cathode to anode through external supply
C) Cathode to anode through internal supply
D) Anode to cathode through internal supply
Answer: C
Explanation: Electrons will move from anode to cathode in the external circuit. And during electrolysis, the negatively charged species (anions) move towards anode and get discharged and therefore we can say that the electrons move internally from cathode to anode.
For more explanation go to What is electrolysis in chemistry?
Question-15) Which of the following statements is incorrect?
A) Electrode is a conductor used in an electrolytic cell to conduct electricity into and out of an electrolyte .
B) The metallic electrode which does not take part in an electrolytic reaction is referred to as inert electrode.
C) Hydroxyl ion takes participation in reaction at anode while sulphate ion does not take participation during electrolysis of acidified water.
D) Carbon is a non-metal. So it cannot be used in electrolytic cell.
Answer: D
Explanation: Graphite form of carbon is a good electrical conductor and hence can be used as electrode.