Surface chemistry deals with the study of phenomena that occur at the surfaces or interfaces of substances, like adsorption, heterogeneous catalysis, formation of colloids, corrosion, crystallization, dissolution, electrode processes, chromatography etc. Surface chemistry finds its applications in industry as well as in daily life.
The physical boundary of any condensed phase like liquid or solid is considered as surface. It separates one phase from the other. It can be considered as series of points which make a plane or layer where one phase ends and the other begins. The surface may be uni-layered or multi-layered.
The interfaces that exist between two immiscible liquids like oil and water; between a metal and a gas like platinum and hydrogen; a liquid and a gas etc., are some examples.
The interfaces between two phases can be represented as: phase1/phase2 or phase1-phase2.
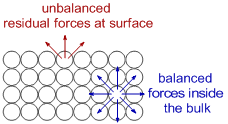
The atoms at the surface show unique properties that are different from those of atoms in the bulk due to unbalanced forces of attraction which are otherwise known as residual forces.
The atoms in the bulk are surrounded in all directions and hence the forces of attraction are balanced. However the forces of attraction at the surface are unbalanced since they are only attracted from inside. The forces of attraction acting outside are not balanced and hence are known as residual forces, which give unique properties to the surface.

Since the formation of a surface requires breaking of attractions between constituent particles, which is an endothermic process all the surfaces are relatively unstable and involves positive free energy of formation. Surfaces contribute positive free energy to the total system.

Hence systems try to minimize this unfavorable free energy at the surfaces, otherwise known as surface tension, in following ways.
i) By reducing the surface area.
ii) By altering the spatial arrangement of particles at the surface.
iii) By making attractions with particles of other systems.
Most of surface chemistry involves the interaction of surfaces of one system with the particles of other system. Surfaces play active role in catalysis, colloid formation, electrode reactions, chromatography etc. In the current chapter we deal with the surface phenomena and related topics like:
2) Catalysis
3) Colloids