(IIT JEE 1996)
a) O2 and H2
b) S2O82-, Na
c) O2, Na
d) S2O82-, H2
Logic & Solution:
* In dilute aqueous solutions, only water undergoes electrolysis to give O2 at anode and H2 at cathode.
The reactions that occur during electrolysis are:
At Anode (oxidation of water): 2H2O ------> 4H+ + O2 + 4e-
At Cathode (reduction of water): 4H2O + 4e- -------> 2H2 + 4OH-
________________________________________________________________
Complete reaction: 2H2O -------> 2H2 + O2
________________________________________________________________
Conclusion:
The correct option is 'a'.
Extra information:
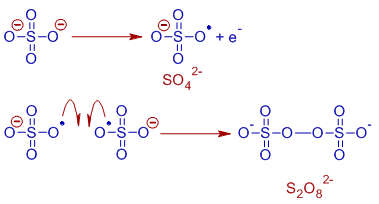
However, at higher concentrations (around 50% Na2SO4), S2O82- is formed at anode instead of O2.
The electrode reaction at anode for the formation persulfate, S2O82- ion from SO42- is shown below:

(IIT JEE 2003)
1) Electrons flow from cathode to anode through the external battery.
2) Electrons flow from cathode to anode within the electrolytic solution.
3) Migration of ions along with oxidation reaction at anode and reduction reaction at cathode.
4) Migration of ions along with oxidation reaction at cathode and reduction reaction at anode.
Logic & solution:
* In electrolytic cell, the electron flow is from anode to cathode through external circuit. Therefore option 1 is incorrect.
* Always oxidation occurs at anode and reduction occurs at cathode.
Conclusion:
Correct statement is: '3'.
(IIT JEE 1990)
a) Cr
b) Al
c) Cu
d) Mg
e) Ag
Logic & solution:
Highly reactive metals with lower reduction potentials than hydrogen are not obtained by electrolysis of aqueous solutions of their salts since their cations cannot be reduced at cathode in presence of water. Instead of reduction of these metal cations, water undergoes reduction.
Al and Mg are reactive metals with lower reduction potentials.
Usually d-block elements are less reactive and hence can be prepared by electrolysis of their aqueous solutions.
Conclusion:
The correct options are: 'b' and 'd'.
1) What is electrolysis?
2) What is an electrolytic cell?
Author: Aditya vardhan Vutturi