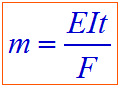
(IIT JEE 1983)
a) atomic number of the cation
b) atomic number of the anion
c) equivalent mass of the electrolyte
d) speed of the cation
Logic:
Faraday's laws are based on the fact that addition or removal of one mole of electrons during reduction or oxidation will liberate or dissolve or deposit one equivalent weight of substance and to achieve this the amount of charge required is equal to charge on one mole of electrons (also know as 1 Faraday and is equal to 96,500 Coulombs).
Conclusion:
The correct option is: 'c'.
FOR ANIMATED INTRODUCTION - WATCH THE FOLLOWING VIDEO .
Faraday’s I Law of Electrolysis: When an electrolyte, either in molten state or solution state is electrolyzed, the amount of substance (m) deposited or dissolved at electrodes is directly proportional to the quantity of electricity (Q) passed through the electrolyte.
Mathematically,
m ∝ Q
or
m = eQ
where, e = electrochemical equivalent
Since e = E/F & Q = I.t
We can also rewrite the equation as:
m = E.I.t/F
Faraday’s II Law of Electrolysis: If the same quantity of electricity is passed through different electrolytic cells, connected in series containing different electrolytic solutions or melts, the amounts of substances deposited or liberated or dissolved are directly proportional to their equivalent weights.
Mathematically, m1/m2 = E1/E2
(IIT JEE 1984)
a) one ampere per second
b) 96,500 Coulombs per second
c) one ampere per hour
d) charge on 1 mole of electrons
Logic:
As already explained in the previous problem, deposition one equivalent of substance requires charge on one mole of electrons.
Conclusion:
The correct option is 'd'.
(IIT JEE 2008)
a) 9.65 x 104 s
b) 28.95 x 104 s
c) 19.3 x 104 s
d) 38.6 x 104 s
Logic:
According to Faraday's first law of electrolysis:
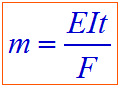
Where:
m = mass of substance
E = equivalent weight of substance
I = current in amperes
t = time required
F = 96,500 Coulombs
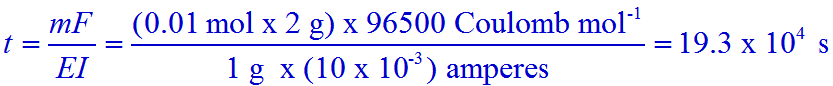
Note:
mass of H2 = m = 0.01 mol x 2 g mol-1
equivalent weight of H2 = E = Atomic weight / valence = 1 g mol-1 / 1 = 1 g mol-1
Current in ampere = I = 10 milliamperes = 10 x 10-3 amperes.
Conclusion:
The correct option is 'c'.
a) 3.0
b) 2.0
c) 0.5
d) 1.0
Logic & solution:
* First we have to calculate the amount of NaCl present in 4.0 molar solution.
The number of moles of NaCl in 500 mL of 4.0 molar solution = Molarity x Volume (in L) = 4.0 x 500 x 10-3 = 2.0 moles.
* The following reaction occurs during electrolysis of aqueous solution of NaCl.
Dissociation of NaCl: 2 NaCl(aq) ------> 2Na+(aq) + 2Cl-(aq)
At cathode: 2H2O + 2e- -------> H2 + 2OH-
At anode: 2Cl-(aq) ---------> Cl2(g) + 2e-
Complete reaction: 2NaCl(aq) + 2H2O ------>H2 + Cl2(g) + 2Na+(aq) + 2OH-(aq)
That means, two moles of NaCl gives one mole of Cl2 gas.
Since there are 2.0 moles of NaCl present in the solution, one mole of Cl2 gas will be evolved at anode upon complete electrolysis.
Conclusion:
The correct option is 'd'.
a) 200 g
b) 446 g
c) 225 g
d) 400 g
Logic & solution:
* When Hg is used as cathode, Na+ will be reduced to Na instead of H2O. Thus formed Na will form sodium amalgam, Na-Hg.
At cathode: Na+ + 1e- + Hg -------> Na-Hg
2.0 moles of NaCl present in the solution contain 2 moles of Na+. Hence two moles of Na-Hg is formed.
The mass of 2 moles of Na-Hg = 2 (23 + 200) = 446 g.
Conclusion:
The correct option is 'b'.
a) 48250 C
b) 96500 C
c) 193000 C
d) 24125 C
Logic & solution:
* Since two moles of electrons are transferred during electrolysis, the total charge required for complete electrolysis is equal to 2 Faraday.
i.e. 2 x 96500 = 193000 C
Conclusion:
The correct option is 'c'.
A) 1 ampere
B) 1 coulomb
C) 1 faraday
D) None of the above
Logic:
For silver, the gram equivalent weight is 108 g. We know that 1 Faraday of electricity is required for deposition of 1 gram equivalent weight of an element.
Conclusion:
Answer: C
6) Faraday's laws of electrolysis will fail when [NCERT 1971]
A) Temperature is increased
B) Inert electrodes are used
C) A mixture of electrolytes is used
D) In none of these cases
Conclusion:
Answer: D
7) Which of the following aqueous solution will result in maximum number of moles of metal deposited on passing same charge?
a) KI
b) ZnSO4
c) AuCl3
d) CaCl2
Conclusion:
Answer: a
8) The quantity of electricity required to liberate 0.01 g equivalent of an element at the electrode is:
A) 9650 coulomb
B) 96500 coulomb
C) 965 coulomb
D) 96.5 coulomb
Hint:
1 g equivalent of element requires 1 Faraday (96500 C). Therefore, 0.01 g equivalent requires 0.01 Faraday or 965 C.
Conclusion:
Answer: C
9) During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is: (NEET 2016)
A) 55 minutes
B) 110 minutes
C) 330 minutes
D) 220 minutes
Solution:
mass of chlorine gas = m = no. of moles x molecular weight = 0.10 mol x 71 g
Equivalent weight of Cl2 gas = E = 35.5 g
Therefore,
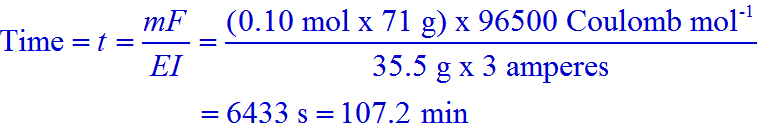
Conclusion:
Answer: B
10) The weight of silver (atomic weight =108) displaced by a quantity of electricity which displaces 5600 mL of O2 at STP will be: (NEET 2014)
A) 5.4 g
B) 10.8 g
C) 54.0 g
D) 108.0 g
Solution:
From Faraday's second law of electrolysis, the no. of equivalents of silver displaced is equal to no. of equivalents of dioxygen gas that is displaced, since same amount of electricity is passed through the solution(s).
i.e. # equivalents of Ag = # equivalents of O2
Now
Equivalent weight of O2 = 8 g
Since, at STP, 32 g of O2 occupy 22400 mL
8 g of O2 occupy 5600 mL at STP
i.e,
# equivalents of O2 displaced = 1
Therefore,
# equivalents of Ag displaced = 1
And we know the equivalent weight of Ag = 108 g (1 gram equivalent)
Conclusion:
Answer: D
1) State Faraday's laws of electrolysis?
2) What are inert electrodes?
| < Previous question | Next question > |